Uric acid and gamma-glutamyl transferase are prognostic indicators in chronic heart failure. Nevertheless, the mechanism underlying the association between uric acid, gamma-glutamyl transferase, and chronic heart failure progression and prognosis remains largely unknown.
MethodsThe association of uric acid and gamma-glutamyl transferase with flow-mediated dilation and echocardiographic indices of cardiac remodeling was addressed in 120 patients with chronic ischemic heart failure. To determine the independent contribution of uric acid and gamma-glutamyl transferase to the flow-mediated dilation and echocardiographic indices of remodeling, a series of multiple linear regression models, based on traditional and nontraditional risk factors impacting upon these parameters, were constructed.
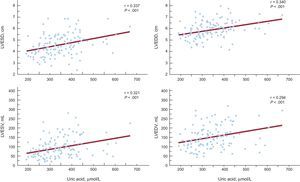
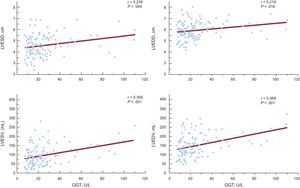
ResultsUric acid, but not gamma-glutamyl transferase, was an independent predictor of flow-mediated dilation. Uric acid was associated with all the echocardiographic indices of left ventricular dysfunction tested in 3 multiple-regression models. Uric acid correlated with left ventricular end-systolic diameter, left ventricular end-diastolic diameter, left ventricular end-systolic volume, and left ventricular end-diastolic volume (r = 0.337; r = 0.340; r = 0.321; r = 0.294; P = .001, respectively). Gamma-glutamyl transferase was an independent predictor of left ventricular end-systolic volume and left ventricular end-diastolic volume, after adjustment for all variables. Gamma-glutamyl transferase correlated with left ventricular end-systolic diameter, left ventricular end-diastolic diameter, left ventricular end-systolic volume, and left ventricular end-diastolic volume (r = 0.238, P = .009; r = 0.219, P = .016; r = 0.359, P < .001; r = 0.369, P = .001, respectively).
ConclusionsSerum uric acid and gamma-glutamyl transferase levels are associated with left ventricular remodeling in patients with chronic ischemic heart failure.
Keywords
Chronic heart failure (CHF) is a highly prevalent syndrome all over the industrialized world and is associated with significant morbidity and mortality. Several types of biomarkers reflecting neurohumoral activation, systemic inflammation, oxidative stress, metabolism and renal dysfunction, as well as anemia, have been shown to be associated with disease severity and progression.1 In addition to brain natriuretic peptide, its derivatives and C-reactive protein, particular attention in CHF prognosis has been paid to 2 inexpensive and easily accessible, highly sensitive laboratory tests, namely, uric acid (UA) and gamma-glutamyl transferase (GGT) determination in plasma. Although elevated plasma levels of UA and GGT are significantly associated with disease severity, their prognostic significance is still controversial in CHF.2,3 Recent work by Poelzl et al4 have indicated a mutual relationship between these biomarkers, since GGT levels were also associated with higher levels of UA and C-reactive protein.4 Nevertheless, the mechanism underlying the association between UA, GGT and CHF progression and prognosis remains largely unknown.
Both GGT and the enzyme xanthine oxidase, one of the putative sources of elevated UA in CHF, are involved in free radical production, followed by enhanced oxidation of biological macromolecules. Free radicals and oxidative stress byproducts are implicated in the key pathophysiological events in the course of CHF progression–endothelial dysfunction and remodeling. Thus, free radicals produced by xanthine oxidase and in GGT-mediated reactions may contribute to sequestration of nitric oxide and the resulting endothelial dysfunction in CHF. Endothelial function, as determined by the dilation of the brachial artery following transient occlusion (flow-mediated vasodilation [FMD]), is inversely correlated with serum UA levels in persons participantswith asymptomatic hyperuricemia associated with essential hypertension,5 as well as in patients with chronic kidney disease.6 Conversely, reduction of UA with a xanthine oxidase inhibitor improves endothelial function in persons participantswith asymptomatic hyperuricemia, as well as in patients with CHF.7,8 Although endothelial dysfunction has been documented in peripheral and coronary arteries in CHF patients,9 the relationship between UA and endothelial function has been investigated in only 1 study, while data on the association between GGT activity and endothelial dysfunction are lacking. In addition, a growing body of evidence suggests an important role of increased oxidative stress in adverse left ventricular remodeling after myocardial infarction.10,11 Our previous study and the others12,13 have shown that the level of the oxidative stress byproduct, malondialdehyde, correlates with the degree of ventricular remodeling in CHF secondary to myocardial infarction and represents an independent predictor of death in these patients. Moreover, recent evidence shows that hyperuricemia contributes to the pathogenesis of myocardial remodeling in experimental heart failure,14–16 Nevertheless, there are no data on the role of UA or GGT in cardiac dysfunction in the clinical settings. We hypothesized that elevated UA and upregulated GGT activity correlate with endothelial dysfunction and ventricular remodeling. Additionally, we hypothesized that potential association of these 2 laboratory markers with endothelial or ventricular dysfunction may be mediated by oxidative stress.
In this translational study, we addressed the association of plasma GGT activity and UA level with FMD and echocardiographic indices of cardiac dysfunction in 120 patients with ischemic CHF and investigated whether these effects are mediated by enhanced oxidative stress.
METHODSStudy GroupThis study enrolled 120 consecutively recruited CHF patients with angiographically confirmed cardiovascular disease at the Bezanijska Kosa Medical Center between 2008 and 2009. The diagnoses of CHF were based on patient history, physical examination, electrocardiography, chest radiology, echocardiography, and coronary angiography. The major inclusion criteria were left ventricular ejection fraction < 45% and steady state of CHF for a 4-week period with conventional pharmacological treatment including diuretics, β-blockers, and angiotensin-converting enzyme inhibitors. Antioxidants and allopurinol were excluded in the previous 2 months. Acute events such as infection, arrhythmia or discontinuation of therapy, which could precipitate manifestations of acute heart failure, were not present in these patients. Regarding decompensation as an exclusion criterion, all New York Heart Association (NYHA) classes III and IV patients were on diuretics and dietary sodium restriction. Patients with severe comorbidity, renal failure, liver disease, and severe disturbances in lung function, as well as those with autoimmune diseases, malignancy, or acute or chronic inflammation were excluded. The age- and sex-matched control group consisted of 69 healthy persons, without no acute or chronic disease or symptoms related to the cardiovascular system. The study was approved by the Ethics Committee of the Faculty of Medicine of Belgrade University. All enrolled patients gave their written informed consent.
Assessment of Cardiac Size and FunctionLeft ventricular ejection fraction, as well as left ventricular end-systolic and end-diastolic dimensions and volumes were determined by 2-dimensional transthoracic echocardiography.17 Left ventricular end-systolic and end-diastolic volumes, as well as left ventricular ejection fraction were estimated using the biplane modified Simpson's rule from apical 2- and 4-chamber views. The dilated ventricle had end-diastolic dimensions ≥ 5.8cm. A left ventricular end-systolic volume of 33 mL to 68 mL (male) and 18 mL to 65 mL (female) and a left ventricular end-diastolic volume of 96 mL to 157 mL (male) and 59 mL to 138 mL (female) were considered as normal values. In addition, Doppler ultrasound and M-mode examinations were carried out. Echocardiograms were performed by the same experienced sonographer using Vivid 7 (GE Medical Systems).
Noninvasive Assessment of Flow-mediated Dilation of the Brachial ArteryEndothelium-dependent and -independent FMD was performed after echocardiographic assessment, using the 13.0MHz linear array transducer (Vivid 7, GE Medical Systems). After a resting period of 15min in the supine position, the transducer was placed 4 cm to 5 cm above the elbow in the longitudinal section for the measurement of the brachial artery basal diameter and flow velocity. A sphygmomanometer cuff was placed on the upper arm and inflated to 250mmHg for 5min, then deflated abruptly, and the second scan was performed 60 s to 90 s later. After 10min of rest, sublingual nitroglycerin (5mg) was administered and the brachial artery was scanned within the next 5min. Diameter measurements were taken at the end of diastole and were calculated at least 3 times. Endothelium-dependent and -independent vasodilations were defined as the percent change in diameter compared with baseline.
Laboratory MethodsBlood samples from CHF patients were taken during outpatient visits. Serum UA, glucose, creatinine, urea, and lipid profile were determined using commercially available kits. Serum GGT activity was measured at 37°C on the day of blood collection by a modular P800 analyzer. The lower limit of detection was 3 U/L while the upper reference limit was set at 38 U/L for women and 65 U/L for men. Plasma malondialdehyde and glutathione peroxidase activity were determined as previously described.12 Neurohormonal status was assessed from plasma brain natriuretic peptide levels, using the brain natriuretic peptide assay Triage® (Biosite Inc.; San Diego, California, United States).
Statistical AnalysisThe difference between 2 arithmetic means was tested by analysis of variance, while the difference between proportions was estimated using the chi-square test. Pearson's correlation coefficient was used to determine the relationship between investigated variables.
To determine the independent contribution of UA and GGT to the echocardiographic indices of remodeling and FMD, we constructed a series of multiple linear regression models based on traditional and nontraditional risk factors impacting upon these parameters. All the factors considered as potentially physiologically relevant for echocardiographic indices of remodeling and FMD were introduced into a standard multivariate linear regression analysis in a 3-step or 4-step procedure, using the enter method. In the first step (model 1), we evaluated the independent influence of UA or GGT, age, sex, body mass index, and smoking on echocardiographic indices of remodeling and FMD. In the second step (model 2), we performed adjustments for age, sex, body mass index, smoking, the presence of diabetes mellitus, and cholesterol. In the third step (model 3), we adjusted the model for the covariates in the second step, plus an additional adjustment for systolic and diastolic blood pressure and creatinine. The fourth step (model 4) was the evaluation of the association between UA and GGT with FMD. In this fourth step, we adjusted the model for the covariates in the third step, plus an additional adjustment for high-sensitivity C-reactive protein.
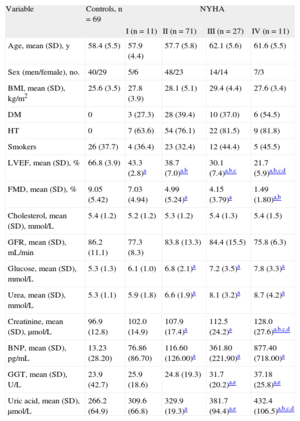
RESULTSGeneral Characteristics of Chronic Heart Failure PatientsThe characteristics of patients and control participants participantsincluded in this study are shown in Table 1. Among CHF patients, no significant differences between groups were observed in terms of age, body mass index, heart rate, and biochemical profile. The relationship between brain natriuretic peptide and NYHA also confirmed brain natriuretic peptide as a quantitative marker of CHF (Table 1). The other clinical characteristics have already been presented in our previous report.12 Left ventricular remodeling and endothelial dysfunction, as a pathophysiological mechanism of CHF progression, were analyzed. All the tested echocardiographic indices of left ventricular dysfunction were significantly higher in NYHA class III-IV patients than in healthy participants and NYHA class I-II patients. The degrees of endothelium-dependent and endothelium-independent vasodilatation (FMD) of the brachial artery get decreased with CHF progression (Table 1).
Clinical Characteristics of the Study Group
| Variable | Controls, n = 69 | NYHA | |||
| I (n = 11) | II (n = 71) | III (n = 27) | IV (n = 11) | ||
| Age, mean (SD), y | 58.4 (5.5) | 57.9 (4.4) | 57.7 (5.8) | 62.1 (5.6) | 61.6 (5.5) |
| Sex (men/female), no. | 40/29 | 5/6 | 48/23 | 14/14 | 7/3 |
| BMI, mean (SD), kg/m2 | 25.6 (3.5) | 27.8 (3.9) | 28.1 (5.1) | 29.4 (4.4) | 27.6 (3.4) |
| DM | 0 | 3 (27.3) | 28 (39.4) | 10 (37.0) | 6 (54.5) |
| HT | 0 | 7 (63.6) | 54 (76.1) | 22 (81.5) | 9 (81.8) |
| Smokers | 26 (37.7) | 4 (36.4) | 23 (32.4) | 12 (44.4) | 5 (45.5) |
| LVEF, mean (SD), % | 66.8 (3.9) | 43.3 (2.8)a | 38.7 (7.0)a,b | 30.1 (7.4)a,b,c | 21.7 (5.9)a,b,c,d |
| FMD, mean (SD), % | 9.05 (5.42) | 7.03 (4.94) | 4.99 (5.24)a | 4.15 (3.79)a | 1.49 (1.80)a,b |
| Cholesterol, mean (SD), mmol/L | 5.4 (1.2) | 5.2 (1.2) | 5.3 (1.2) | 5.4 (1.3) | 5.4 (1.5) |
| GFR, mean (SD), mL/min | 86.2 (11.1) | 77.3 (8.3) | 83.8 (13.3) | 84.4 (15.5) | 75.8 (6.3) |
| Glucose, mean (SD), mmol/L | 5.3 (1.3) | 6.1 (1.0) | 6.8 (2.1)a | 7.2 (3.5)a | 7.8 (3.3)a |
| Urea, mean (SD), mmol/L | 5.3 (1.1) | 5.9 (1.8) | 6.6 (1.9)a | 8.1 (3.2)a | 8.7 (4.2)a |
| Creatinine, mean (SD), μmol/L | 96.9 (12.8) | 102.0 (14.9) | 107.9 (17.4)a | 112.5 (24.2)a | 128.0 (27.6)a,b,c,d |
| BNP, mean (SD), pg/mL | 13.23 (28.20) | 76.86 (86.70) | 116.60 (126.00)a | 361.80 (221,90)a | 877.40 (718.00)a |
| GGT, mean (SD), U/L | 23.9 (42.7) | 25.9 (18.6) | 24.8 (19.3) | 31.7 (20.2)a,e | 37.18 (25.8)a,e |
| Uric acid, mean (SD), μmol/L | 266.2 (64.9) | 309.6 (66.8) | 329.9 (19.3)a | 381.7 (94.4)a,e | 432.4 (106.5)a,b,c,d |
BMI, body mass index; BNP, brain natriuretic peptide; DM, diabetes mellitus; FMD, flow-mediated vasodilation; GFR, glomerular filtration rate; GGT, gamma-glutamyl transferase; HT, hypertension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
Statistically significant difference compared with New York Heart Association class I patients (P < .05).
Statistically significant difference compared with New York Heart Association class II patients (P < .05).
As shown in Table 1, UA levels were significantly elevated in NYHA class II-IV patients compared with healthy participants participants(P < .001). There was a progressive increase of UA from controls to the patients in the worst functional class, with the rise being more pronounced in NYHA class IV (P < .001). A moderate, but still significant correlation was demonstrated for serum UA levels with brain natriuretic peptide (r = –0.361; P = .001), as well as with left ventricular ejection fraction (r = −0.335; P < .001), showing a clear relationship with the severity of myocardial dysfunction. Mean GGT activities in patients in NYHA classes I-II did not differ significantly from the values obtained in controls. However, GGT levels were higher in NYHA class III-IV patients compared with controls (P < .01) as well as in comparison with NYHA class I-II (P < .01) (Table 1). GGT levels did not correlate with brain natriuretic peptide (r = −0.035; P = .707), but a correlation was found between GGT levels and left ventricular ejection fraction (r = −0.259; P = .004).
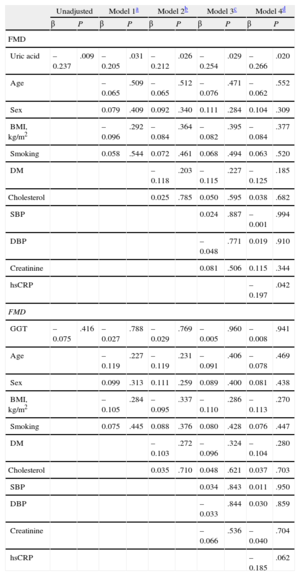
Association of Uric Acid With Flow-mediated Vasodilation in Chronic Heart Failure PatientsThe association between UA and GGT activity and FMD is presented in Table 2. In the stepwise multiple-regression analyses, UA was a statistically significant independent predictor of FMD in all 4 models tested (model 1: β = −0.205, P = .031; model 2: β = −0.212, P = .026; model 3: β = −0.254, P = .029; model 4: β = −0.266, P = .020) (Table 2). GGT was not a statistically significant predictor of FMD (Table 2).
Multiple Linear Regression Models on Association of Flow-mediated Dilation With Uric Acid and Gamma-glutamyl Transferase
| Unadjusted | Model 1a | Model 2b | Model 3c | Model 4d | ||||||
| β | P | β | P | β | P | β | P | β | P | |
| FMD | ||||||||||
| Uric acid | –0.237 | .009 | –0.205 | .031 | –0.212 | .026 | –0.254 | .029 | –0.266 | .020 |
| Age | –0.065 | .509 | –0.065 | .512 | –0.076 | .471 | –0.062 | .552 | ||
| Sex | 0.079 | .409 | 0.092 | .340 | 0.111 | .284 | 0.104 | .309 | ||
| BMI, kg/m2 | –0.096 | .292 | –0.084 | .364 | –0.082 | .395 | –0.084 | .377 | ||
| Smoking | 0.058 | .544 | 0.072 | .461 | 0.068 | .494 | 0.063 | .520 | ||
| DM | –0.118 | .203 | –0.115 | .227 | –0.125 | .185 | ||||
| Cholesterol | 0.025 | .785 | 0.050 | .595 | 0.038 | .682 | ||||
| SBP | 0.024 | .887 | –0.001 | .994 | ||||||
| DBP | –0.048 | .771 | 0.019 | .910 | ||||||
| Creatinine | 0.081 | .506 | 0.115 | .344 | ||||||
| hsCRP | –0.197 | .042 | ||||||||
| FMD | ||||||||||
| GGT | –0.075 | .416 | –0.027 | .788 | –0.029 | .769 | –0.005 | .960 | –0.008 | .941 |
| Age | –0.119 | .227 | –0.119 | .231 | –0.091 | .406 | –0.078 | .469 | ||
| Sex | 0.099 | .313 | 0.111 | .259 | 0.089 | .400 | 0.081 | .438 | ||
| BMI, kg/m2 | –0.105 | .284 | –0.095 | .337 | –0.110 | .286 | –0.113 | .270 | ||
| Smoking | 0.075 | .445 | 0.088 | .376 | 0.080 | .428 | 0.076 | .447 | ||
| DM | –0.103 | .272 | –0.096 | .324 | –0.104 | .280 | ||||
| Cholesterol | 0.035 | .710 | 0.048 | .621 | 0.037 | .703 | ||||
| SBP | 0.034 | .843 | 0.011 | .950 | ||||||
| DBP | –0.033 | .844 | 0.030 | .859 | ||||||
| Creatinine | –0.066 | .536 | –0.040 | .704 | ||||||
| hsCRP | –0.185 | .062 | ||||||||
FMD, flow-mediated vasodilation; GGT, gamma-glutamyl transferase; BMI, body mass index; DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high sensitivity-C-reactive protein.
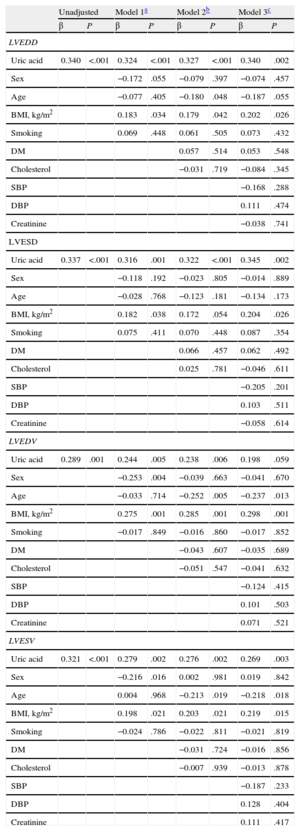
Stepwise multiple-regression analyses of the association between UA level and left ventricular end-diastolic diameter revealed that UA remained a statistically significant independent predictor associated with left ventricular end-diastolic diameter in all 3 models (model 1: β = 0.324, P < .001; model 2: β = 0.327, P < .001; model 3: β = 0.340, P = .002) (Table 3). UA was also a statistically significant independent predictor of left ventricular end-systolic diameter after adjustment for all variables (model 1: β = 0.316, P = .001; model 2: β = 0.322, P < .001; model 3: β = 0.345, P = .002), as well as of left ventricular end-systolic volume (model 1: β = 0.279, P = .002; model 2: β = 0.276, P = .002; model 3: β = 0.269, P = .003) (Table 3). In the stepwise multiple-regression analyses of the association between UA and left ventricular end-diastolic volume, UA remained a statistically significant independent predictor of left ventricular end-diastolic volume in model 1 (β = 0.244, P = .005) and model 2 (β = 0.238, P = .006), but the significance was lost in model 3 (β = 0.198, P = .059) (Table 3). The UA level correlated significantly with all echocardiographic indices of left ventricular dysfunction: left ventricular end-systolic and end-diastolic diameters, left ventricular end-systolic and end-diastolic volumes (r = 0.337, P < .001; r = 0.340, P < .001; r = 0.321, P < .001; r = 0.294, P = .001, respectively) (Figure 1).
Multiple Linear Regression Models on Association Between Uric Acid and Echocardiographic Indices of Remodeling
| Unadjusted | Model 1a | Model 2b | Model 3c | |||||
| β | P | β | P | β | P | β | P | |
| LVEDD | ||||||||
| Uric acid | 0.340 | <.001 | 0.324 | <.001 | 0.327 | <.001 | 0.340 | .002 |
| Sex | −0.172 | .055 | −0.079 | .397 | −0.074 | .457 | ||
| Age | −0.077 | .405 | −0.180 | .048 | −0.187 | .055 | ||
| BMI, kg/m2 | 0.183 | .034 | 0.179 | .042 | 0.202 | .026 | ||
| Smoking | 0.069 | .448 | 0.061 | .505 | 0.073 | .432 | ||
| DM | 0.057 | .514 | 0.053 | .548 | ||||
| Cholesterol | −0.031 | .719 | −0.084 | .345 | ||||
| SBP | −0.168 | .288 | ||||||
| DBP | 0.111 | .474 | ||||||
| Creatinine | −0.038 | .741 | ||||||
| LVESD | ||||||||
| Uric acid | 0.337 | <.001 | 0.316 | .001 | 0.322 | <.001 | 0.345 | .002 |
| Sex | −0.118 | .192 | −0.023 | .805 | −0.014 | .889 | ||
| Age | −0.028 | .768 | −0.123 | .181 | −0.134 | .173 | ||
| BMI, kg/m2 | 0.182 | .038 | 0.172 | .054 | 0.204 | .026 | ||
| Smoking | 0.075 | .411 | 0.070 | .448 | 0.087 | .354 | ||
| DM | 0.066 | .457 | 0.062 | .492 | ||||
| Cholesterol | 0.025 | .781 | −0.046 | .611 | ||||
| SBP | −0.205 | .201 | ||||||
| DBP | 0.103 | .511 | ||||||
| Creatinine | −0.058 | .614 | ||||||
| LVEDV | ||||||||
| Uric acid | 0.289 | .001 | 0.244 | .005 | 0.238 | .006 | 0.198 | .059 |
| Sex | −0.253 | .004 | −0.039 | .663 | −0.041 | .670 | ||
| Age | −0.033 | .714 | −0.252 | .005 | −0.237 | .013 | ||
| BMI, kg/m2 | 0.275 | .001 | 0.285 | .001 | 0.298 | .001 | ||
| Smoking | −0.017 | .849 | −0.016 | .860 | −0.017 | .852 | ||
| DM | −0.043 | .607 | −0.035 | .689 | ||||
| Cholesterol | −0.051 | .547 | −0.041 | .632 | ||||
| SBP | −0.124 | .415 | ||||||
| DBP | 0.101 | .503 | ||||||
| Creatinine | 0.071 | .521 | ||||||
| LVESV | ||||||||
| Uric acid | 0.321 | <.001 | 0.279 | .002 | 0.276 | .002 | 0.269 | .003 |
| Sex | −0.216 | .016 | 0.002 | .981 | 0.019 | .842 | ||
| Age | 0.004 | .968 | −0.213 | .019 | −0.218 | .018 | ||
| BMI, kg/m2 | 0.198 | .021 | 0.203 | .021 | 0.219 | .015 | ||
| Smoking | −0.024 | .786 | −0.022 | .811 | −0.021 | .819 | ||
| DM | −0.031 | .724 | −0.016 | .856 | ||||
| Cholesterol | −0.007 | .939 | −0.013 | .878 | ||||
| SBP | −0.187 | .233 | ||||||
| DBP | 0.128 | .404 | ||||||
| Creatinine | 0.111 | .417 | ||||||
BMI, body mass index; DM, diabetes mellitus; DBP, diastolic blood pressure; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; SBP, systolic blood pressure.
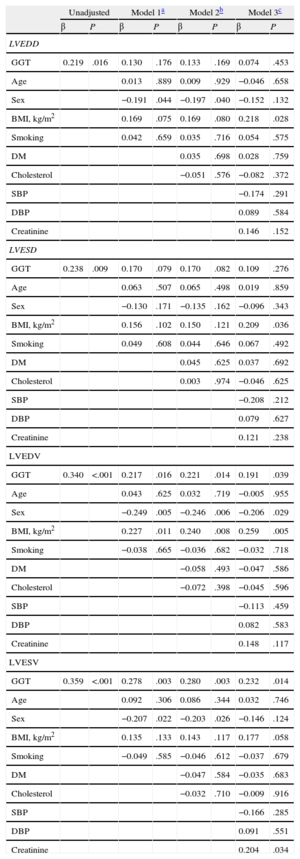
Gamma-glutamyl transferase was a significant predictor of left ventricular end-diastolic and end-systolic diameters in unadjusted regression analyses (β = 0.219, P = .016; β = 0.238, P = .009; respectively), but after adjustments in 3 separate multiple-regression models, its effect on diameters was nonsignificant (Table 4). In the stepwise multiple-regression analyses of left ventricular end-diastolic volume, GGT was a statistically significant independent predictor that correlated with left ventricular end-diastolic volume in all 3 models (model 1: β = 0.217, P = .016; model 2: β = 0.221, P = .014; model 3: β = 0.191, P = .039) (Table 4). GGT was a statistically significant independent predictor of left ventricular end-systolic volume after adjustment for all variables (model 1: β = 0.278, P = .003; model 2: β = 0.280, P = .003; model 3: β = 0.232, P = .014) (Table 4). Moreover, GGT activity significantly correlated with left ventricular end-systolic and end-diastolic diameters, left ventricular end-systolic and end-diastolic volumes (r = 0.238, P = .009; r = 0.219, P = .016; r = 0.359, P < .001; r = 0.369, P = .001, respectively) (Figure 2).
Multiple Linear Regression Models on Association Between Gamma-glutamyl Transferase and Echocardiographic Indices of Remodeling
| Unadjusted | Model 1a | Model 2b | Model 3c | |||||
| β | P | β | P | β | P | β | P | |
| LVEDD | ||||||||
| GGT | 0.219 | .016 | 0.130 | .176 | 0.133 | .169 | 0.074 | .453 |
| Age | 0.013 | .889 | 0.009 | .929 | −0.046 | .658 | ||
| Sex | −0.191 | .044 | −0.197 | .040 | −0.152 | .132 | ||
| BMI, kg/m2 | 0.169 | .075 | 0.169 | .080 | 0.218 | .028 | ||
| Smoking | 0.042 | .659 | 0.035 | .716 | 0.054 | .575 | ||
| DM | 0.035 | .698 | 0.028 | .759 | ||||
| Cholesterol | −0.051 | .576 | −0.082 | .372 | ||||
| SBP | −0.174 | .291 | ||||||
| DBP | 0.089 | .584 | ||||||
| Creatinine | 0.146 | .152 | ||||||
| LVESD | ||||||||
| GGT | 0.238 | .009 | 0.170 | .079 | 0.170 | .082 | 0.109 | .276 |
| Age | 0.063 | .507 | 0.065 | .498 | 0.019 | .859 | ||
| Sex | −0.130 | .171 | −0.135 | .162 | −0.096 | .343 | ||
| BMI, kg/m2 | 0.156 | .102 | 0.150 | .121 | 0.209 | .036 | ||
| Smoking | 0.049 | .608 | 0.044 | .646 | 0.067 | .492 | ||
| DM | 0.045 | .625 | 0.037 | .692 | ||||
| Cholesterol | 0.003 | .974 | −0.046 | .625 | ||||
| SBP | −0.208 | .212 | ||||||
| DBP | 0.079 | .627 | ||||||
| Creatinine | 0.121 | .238 | ||||||
| LVEDV | ||||||||
| GGT | 0.340 | <.001 | 0.217 | .016 | 0.221 | .014 | 0.191 | .039 |
| Age | 0.043 | .625 | 0.032 | .719 | −0.005 | .955 | ||
| Sex | −0.249 | .005 | −0.246 | .006 | −0.206 | .029 | ||
| BMI, kg/m2 | 0.227 | .011 | 0.240 | .008 | 0.259 | .005 | ||
| Smoking | −0.038 | .665 | −0.036 | .682 | −0.032 | .718 | ||
| DM | −0.058 | .493 | −0.047 | .586 | ||||
| Cholesterol | −0.072 | .398 | −0.045 | .596 | ||||
| SBP | −0.113 | .459 | ||||||
| DBP | 0.082 | .583 | ||||||
| Creatinine | 0.148 | .117 | ||||||
| LVESV | ||||||||
| GGT | 0.359 | <.001 | 0.278 | .003 | 0.280 | .003 | 0.232 | .014 |
| Age | 0.092 | .306 | 0.086 | .344 | 0.032 | .746 | ||
| Sex | −0.207 | .022 | −0.203 | .026 | −0.146 | .124 | ||
| BMI, kg/m2 | 0.135 | .133 | 0.143 | .117 | 0.177 | .058 | ||
| Smoking | −0.049 | .585 | −0.046 | .612 | −0.037 | .679 | ||
| DM | −0.047 | .584 | −0.035 | .683 | ||||
| Cholesterol | −0.032 | .710 | −0.009 | .916 | ||||
| SBP | −0.166 | .285 | ||||||
| DBP | 0.091 | .551 | ||||||
| Creatinine | 0.204 | .034 | ||||||
BMI, body mass index; DM, diabetes mellitus; DBP, diastolic blood pressure, GGT, gamma-glutamyl transferase; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; SBP, systolic blood pressure.
Correlations of gamma-glutamyl transferase with echocardiographic indices of remodeling. GGT, gamma-glutamyl transferase; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume.
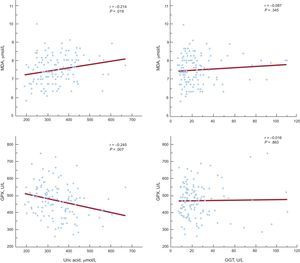
To assess whether an increased UA level and GGT activity might be related to enhanced oxidative stress, we correlated these parameters with the plasma malondialdehyde level and glutathione peroxidase activity. The UA level was significantly associated with both malondialdehyde concentration and glutathione peroxidase activity (r = 0.214, P = .019 and r = −0.245, P = .007, respectively). However, plasma GGT activity was not associated with the examined markers of oxidative stress (Figure 3).
DISCUSSIONAlthough endothelial dysfunction has been documented in peripheral and coronary arteries in CHF patients,9 only 1 study in 38 mostly male participants with NYHA class II and III CHF has found an association between UA and endothelial function. Because the strict inclusion criteria (FMD < 8%) in this study could impair the generalization of the results to a less severe CHF population, we hypothesized that, in the early stages of CHF, increased serum UA levels, as an indicator of xanthine oxidase activation, could be used as a risk marker while, in the later phases, UA could act as an antioxidant, having a protective action on extracellular antioxidant enzymes and improving endothelial function. However, the results of the current study, which included 3 times more participants with various stages of CHF, have shown an inverse correlation between UA and FMD, while the activity of the antioxidant enzyme glutathione peroxidase was inversely correlated with UA. These results are biologically plausible, given that the main source of elevated UA in CHF is xanthine oxidase, the enzyme that produces superoxide or hydrogen peroxide as byproducts of the terminal steps of purine metabolism in the presence of hypoxia. Under these conditions, an increase in superoxide may inactivate nitric oxide,18 suggesting an important underlying mechanism in the development of vascular endothelial dysfunction in CHF, which contributes to systemic vasoconstriction and increased cardiac loading.
Cardiac remodeling is an unfavorable prognostic factor associated with myocardial hypertrophy, fibrosis, and ventricular dysfunction after myocardial infarction.19 The proposed mechanism causing a decrease in myocardial contractility is the cell damage produced by oxygen-free radicals, leading to peroxidation of membrane phospholipids, which can result in an increase in membrane fluidity, increasing permeability and loss of membrane integrity.20,21 In this study, we have shown that the UA level correlates with the degree of myocardial dysfunction, based on a linear regression analysis of the association between UA level and the echocardiographic indices of left ventricular remodeling. This effect of UA on remodeling seems to be at least partially mediated by enhanced lipid peroxidation, since UA correlated significantly with the lipid peroxidation byproduct malondialdehyde and antioxidant glutathione peroxidase activity. Xanthine oxidase expression and activity were found to be markedly increased in the tissue 2mm surrounding the infarct area22 in a mice model of myocardial infarction. In the heart, xanthine oxidase is localized solely in the capillary endothelium.23 Therefore, the UA generated in hypoxic states originates from capillary endothelial cells, rather than from the myocardium,24 and hyperuricemia in heart failure may reflect the metabolic effects of hypoxia on the microvasculature. The correlation between UA and left ventricular dysfunction found in the current study confirms the results of in vitro studies that have shown that reactive oxygen species production by xanthine oxidase leads to a depression of the excitation-contraction coupling mechanism in cardiac muscle.25,26 These effects would induce a decrease in cardiac contractility and in the rate of cardiac muscle relaxation. In addition to xanthine oxidase, hyperuricemia itself can also influence free radical production and myocardial remodeling. Chen et al16 have shown that hyperuricemia induced by oxonic acid stimulates myocardial superoxide production, resulting in enhanced endothelin-1-induced ventricular remodeling in infarcted rats. Furthermore, treatment of hyperuricemic rats with allopurinol and benzbromarone, UA-lowering agents, attenuated remodeling after myocardial infarction. However, Cicoira et al27, who evaluated the effects of elevated UA levels on cardiac function in 150 CHF patients resulting from dilated cardiomyopathy of diverse etiology, found a significant correlation with diastolic dysfunction, but not with markers of systolic function or left ventricular volumes. The discrepancies between these findings and our own may be a consequence of differences in the study cohort, which was homogenous in our study with respect to the cause of CHF. We showed a positive correlation between UA levels and both malondialdehyde and echocardiographic indices of remodeling. Although these findings do not support a causal relationship between UA and remodeling, they do suggest that UA could contribute to the increased oxidative stress present in CHF. Further experimental trials should be conducted to clarify the real impact of UA on the physiology of cardiovascular disease.
Several population-based studies have consistently shown that serum GGT activity, mostly within normal ranges, are strongly associated with most cardiovascular risk factors. Although the mechanism underlying this association remains largely unknown, several explanations for this phenomenon have been proposed, including hepatic congestion, increased free radical production and inflammation.4 In this study, we addressed the potential involvement of GGT in the pathogenesis of endothelial dysfunction and left ventricular remodeling in heart failure, since both of these events are related to oxidative stress. Specifically, membrane bound GGT is involved in degradation of the antioxidant glutathione, which ultimately results in the amino acids cysteine and glycine.28 The reactive thiol of cysteinyl-glycine can generate superoxide anion radicals and hydrogen peroxide through its interaction with free iron.29 These GGT-mediated reactions have been shown to catalyze the oxidation of low-density lipoproteins, which may contribute to oxidative events influencing plaque evolution and rupture.30 Indeed, a recent study has shown an association of GGT with coronary atherosclerosis progression in patients with ischemic heart disease on statin treatment.31 Coronary artery disease and myocardial infarction were the causes of CHF in this study. Cysteine and glycine constitute the precursors of intracellular glutathione. Hence, GGT also provides a supply for uptake and reutilization in intracellular glutathione synthesis. In this way, GGT serves as a rescue enzyme for cellular glutathione synthesis and thus plays an important role in antioxidant defense systems. Accordingly, it has been suggested that an increase in serum GGT activity could be used as a marker for increased oxidative stress in humans.32,33 Although the results of our study have indicated a relationship between cardiac dysfunction and GGT activity, it seems that it is not related to either increased lipid oxidation or impairment of antioxidant activity. Thus, in contrast to UA, serum GGT activity was not associated with the biomarkers of oxidative stress in CHF. Therefore, it may be speculated that elevated GGT in heart failure is only a part of the cholestatic profile of laboratory elevations seen in these patients, secondary to hepatic congestion. Nevertheless, the question of the mechanisms involved in the association between GGT and cardiac dysfunction should be addressed in future in vitro and animal studies.
The last decade has seen a significant advance in the pathophysiology of heart failure. As a result, many different diagnostic and prognostic markers have been proposed and few have shown clear clinical use.34,35 Among those, serum UA seems to fulfill these criteria. First, accurate, repeated measurements of UA are available to the clinician at a reasonable cost and with short turnaround times; second, the determination of serum UA provides information that is not already available from a careful clinical assessment; and finally, knowing the measured UA concentration should aid in medical decision-making. The current study provides a moderate correlation between levels of UA and indices of remodeling. On the other hand, UA and other currently assessable markers of CHF lack cardiac specificity, and their levels can be influenced by both systemic inflammatory and infective processes, which occur frequently in heart failure patients. Until now, several new biomarkers with a plausible biological link with heart failure pathophysiology have been identified. Among them, ST-2 and galectin share the ability to define the severity of the ongoing ventricular remodeling process.34,36 However, their clinical use should be confirmed in large-scale studies.
LimitationsThe limitations of this study include its cross-sectional design and rather small sample size, especially in certain NYHA subgroups. The cross-sectional design of the study precludes determining a causal relationship and the prognostic value of these determinations. Therefore, these findings warrant validation in further prospective studies with larger numbers of patients in advanced NYHA classes in cardiac dysfunction.
CONCLUSIONSIn this study, we show that serum levels of UA and GGT activity are associated with echocardiographic indices of left ventricular remodeling in patients with CHF secondary to ischemic coronary disease. Regarding endothelial function, only serum UA showed an inverse correlation with endothelium-dependent vasodilation in CHF. The effects of UA on endothelial function and left ventricular dysfunction may be at least partially explained by its relationship with oxidative stress markers such as malondialdehyde and glutathione peroxidase activity.
FundingThis work was supported by the grant 175052 from the Serbian Ministry of Education, Science and Technological Developmental.
CONFLICTS OF INTERESTNone declared.