Keywords
INTRODUCTION
Postinfarction ventricular septal defects (PIVSD) are a serious complication that is usually treated by surgery. Percutaneous closure using Amplatzer devices is a recent alternative, though the references concern just single cases or small series.1-5 The aim of this paper is to present our experience with this technique.
METHODS
From December 2000 to June 2006 we attempted percutaneous closure of PIVSD with Amplatzer devices in 19 patients (two with recanalization after surgical closure and 17 with primary PIVSD). The techniques used have been described previously.1-5 Table 1 shows the clinical data of the patients. All the patients were referred from other centers and the mean age of the patients was 65.5 years. The procedure was carried out 2-3 weeks after the infarction in 3 acute phase patients, from 3.5 to 12 weeks after infarction in 15 subacute phase patients (2 postsurgical) and almost 1 year later in 1 chronic phase patient. Echocardiographic study showed a simple defect in 13 patients with primary PIVSD (direct shunt) and a complex form in four patients (dissection of the interventricular septum).6 All the patients had symptomatic heart failure: 16 required inotropic support and 5 needed an aortic counterpulsation balloon. Coronary angiogram performed prior to percutaneous closure showed multivessel coronary artery disease in 10 patients and stents were implanted in three patients. The artery causing the PIVSD ruptured in 6 cases. The crucial point of the procedure was inserting the probe through the interventricular septum: the defect was approached from the right ventricle in 14 cases and from the left in eight (creating an arteriovenous loop). The closure was done via the right internal jugular vein (RIJV) or the right femoral vein (RFV) under echocardiographic and fluoroscopic control, choosing the access that caused the least curve in order to avoid a possible kinking of the sheath. In all except the last three patients the diameter of the PIVSD was measured with a balloon.2 Depending on the morphology of the septum and the properties of the various devices, the following Amplatzers were chosen: 17 atrial septal occluders (ASO), two muscular ventricular septal defect occluders (MVSDO) and two postinfarction muscular ventricular septal defect occluders (PIMVSDO), always with a larger size than the defect (1-10 mm). The residual shunt was evaluated after the closure by echocardiography after suspending the infusion of heparin, generally one week after the procedure.
RESULTS
The main data concerning the procedures and the results are shown in Table 2. A total of 22 operations were carried out in 19 patients, with no case of peripheral embolisms in the devices. Two sessions were necessary in three cases (2 with a double PIVSD) as the first attempt was ineffective. The implantation was satisfactory in 14 patients: 11 with primary PIVSD in the subacute phase, 1 with chronic PIVSD and 2 postsurgical (Figures A and B), but in none of the acute phase patients. The defect could not be closed in two patients with a large PIVSD (Table 2, patients 5 and 9). In the first case, already reported,2 the defect could not be traversed with any wire, and in the second case the sheath kinked and had to be withdrawn. In another 2 patients with acute PIVSD (cases 7 and 14) atrial septal occluders of 24 and 30 mm traversed the defect freely and both patients later underwent surgery, at which time the PIVSD was seen to be enlarged. In another acute phase patient (case 13) the sheath, introduced via the RIJV, kinked and another attempt was made via the RFV, but the patient suffered cardiac tamponade and later died. Despite the initial success, four patients with acute phase PIVSD (cases 1, 8, 15 and 18) in unstable hemodynamic conditions (2 required aortic counterpulsation) died due to multiorgan failure 1-3 weeks after implantation of the device. The mean fluoroscopic time was 49 min (interval, 10-120 min). Of the 14 cases with a satisfactory result, complete closure of the defect was achieved in two, a small residual shunt remained in nine, a moderate shunt in one and an important shunt in two. These latter 2 later underwent surgery for complete closure, with the percutaneous procedure acting as a bridge to surgery and their general status improving considerably (patients 10 and 11). The following complications were noted during the procedure: 2 transitory grade III atrio-ventricular blocks, 5 cases with episodes of ventricular tachycardia/fibrillation that required defibrillation, and transitory hemolysis that disappeared after 2 weeks.
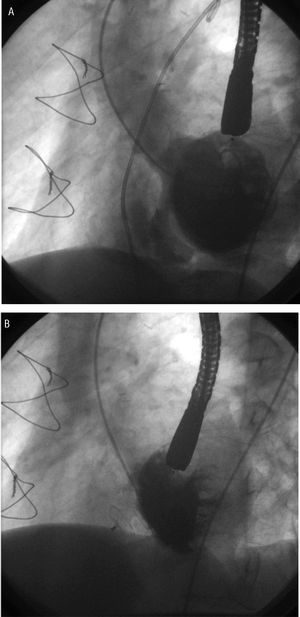
Figure. Transcatheter closure of a postinfarction ventricular septal defect (PIVSD) recanalized after earlier surgery. Left ventriculogram on long axis during early diastole. A: important left-right shunt through an oblique canal of the PIVSD. B: Amplatzer atrial septal occluder closing the PIVSD.
DISCUSSION
According to the classification of Edwards et al,6 two types of PIVSD exist, simple and complex, and the defect can be either very large or multiple, with the possibility of increasing over time. Patients with PIVSD are generally in an unstable clinical position, with acute hemodynamic changes that are poorly tolerated because of a reduction in ventricular function. The optimal treatment strategy is still under discussion.7 Percutaneous closure during the acute phase after the infarction is a high risk procedure with a high likelihood of failure, as occurred in all our acute phase patients in whom it was attempted. In these cases, even the oversized devices easily traversed the defects, which may well have caused additional widening. Another additional risk involves the theoretical formation of a systemic or pulmonary embolism in part of the necrotic tissue, which is very fragile at the edge of the defect. Our study shows that better results can be obtained when the procedure is delayed to at least 3,5 weeks after the infarction, when the scar tissue is already formed and can provide a better support for the device. The irregular edges of the PIVSD may be an additional cause of the residual shunt, unlike the smooth edges that are present in patients with a congenital defect.8 A postsurgical recurrent, residual defect occurs in about 10% of survivors of PIVSD in the acute phase. In patients with residual or recurrent defects, natural survivors or after surgical repair, the tissue surrounding the defect is firmer and the size relatively constant. These patients are therefore candidates for percutaneous closure, thereby avoiding open heart surgery.
One important conclusion from this study is that, in many subacute phase patients, either chronic or postsurgical, the edges of the PIVSD are thinner and the ASO devices fit better, although the technique is usually very laborious. We also noted that the current sheath of the Amplatzer occluder fails to provide the necessary combination of support and flexibility. This can result in kinking, which impedes the correct implantation of the device, as happened in two of our patients. It has recently been suggested that only simple PIVSD are suitable for percutaneous closure.4 In general, we agree with this criteria, though exceptions can be found, as was seen in our patients 11 and 19. The Cribiform device might be the therapeutic answer to acute or multiple PIVSD.5 Another option concerns the use of double umbrella devices, such as the Starflex or occlusion with a transcatheter patch.9
Percutaneous closure of the PIVSD in chronic patients with a residual shunt after prior surgery is possibly the treatment of choice. The Amplatzer atrial septal occluder is probably, in most cases, the most suitable device available. Patients with acute phase PIVSD (fewer than 3.5 weeks after the infarct), however, are not good candidates for percutaneous closure, at least with the currently available Amplatzer devices, which require a new design. In the subacute phase, severe left ventricular failure and multiorgan dysfunction sometimes inhibit clinical improvement, even though the initial immediate result is satisfactory.
Correspondence: Dr. J. Bialkowski.
Silesian Center for Heart Disease,
ul. Szpitalna 2, 41-800 Zabrze, Poland,
E-mail: jabi_med@poczta.onet.pl
Received October 24, 2006.
Accepted for publication February 15, 2007.