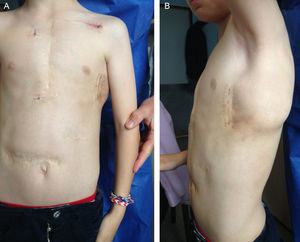
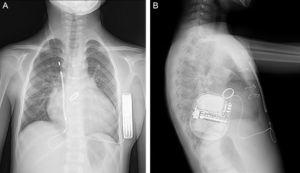
A 9-year-old boy, weighing 24kg and measuring 130cm in height, was referred to us for implantation of a totally subcutaneous implantable cardioverter-defibrillator (ICD). He underwent surgery for truncus arteriosus type I at age 20 days, with closure of the ventricular septal defect, truncal valvuloplasty and placement of a right ventricle-to-pulmonary artery Contegra® conduit. At age 3 years, the patient required reintervention. A mechanical aortic prosthesis was implanted and the conduit was changed. At age 8 years, he had a sudden cardiac death episode while playing football, and was successfully resuscitated by emergency teams. The prosthesis was working correctly, the conduit showed no stenosis and left ventricular function was normal. An electrophysiological study was carried out, with induced ventricular fibrillation. It was decided to implant a single-chamber defibrillator in the left infraclavicular position (Energen™ DAI Boston Scientific Inc.; Natick, Massachusetts, United States) with a transvenous electrode in the right ventricular apex. The generator and electrode had to be removed 8 months later due to device infection. We therefore decided to implant a totally subcutaneous ICD system consisting of a generator and lead (Cameron Health, Boston Scientific Inc.). Beforehand, we checked that the patient was a suitable candidate in terms of TQRS morphology and body size. We performed the procedure in the electrophysiological laboratory under general anesthesia, implanting the subcutaneous ICD along the anterior axillary line, between the serratus anterior and latissimus dorsi muscles (Figure 1). We placed the subcutaneous electrode in the right parasternal region, because this is the best detection zone. A defibrillation test at 65J was successful. We programmed a shock zone at 220 bpm and a conditional shock zone at 200 bpm. A chest X-ray before discharge showed that the electrode and generator were in the correct position (Figure 2). At the 4-month follow-up, the patient had experienced no shocks or other complications.
Subcutaneous ICD appears to be a valid alternative to the conventional transvenous system for patients not requiring pacing for bradycardia, resynchronization or antitachycardia treatment. More than 3500 devices have been implanted worldwide since they appeared in the market. Rates for successful cardioversion in ventricular arrhythmias, complications and inappropriate shocks are similar in subcutaneous ICD and conventional transvenous defibrillators alike.1,2 Experience in pediatric patients is much more limited, as reflected in the few cases published to date.3,4 As far as we are aware, our patient is the youngest and smallest in terms of height and weight reported to date, which made us question the suitability of subcutaneous ICD in this body size. Furthermore, the patient had complex congenital heart disease, had already undergone several surgical procedures, and had suffered an infection of a previous conventional transvenous defibrillator. The combination of these factors made this case unique. We believe that subcutaneous ICD is an excellent alternative for children with these characteristics, because it avoids the long-term complications of transvenous electrodes. A recently published case series of children and young adults5 reported a 14% incidence of electrode failure 2 years after implantation. The failure rate increased in correlation with younger patient age. In children, there is still understandable doubt about the durability of a totally subcutaneous system. Children's physical activity and predisposition to accidents could limit the benefits of this system, causing inappropriate shocks through myopotentials triggered by external agents not described to date in transvenous systems.6