Although several investigators have observed that the incidence of cardiac arrest is diminishing,1 the overall survival remains disappointingly low at 10% or less.2 Cardiovascular disease remains responsible for 41% of all deaths in Europe. Cardiac arrest is usually the catastrophic first symptom of a heart attack.
Recently, several organizations have managed to improve their chain of survival so that up to 61% of cardiac arrest victims presenting with ventricular fibrillation (VF) or ventricular tachycardia (VT) can be resuscitated successfully,3 but systematic reviews have demonstrated that outcome differs dramatically between regions: published survival figures range between 6% and 31% for all cardiac arrests and between 8% and 43% for VF/VT arrests.4, 5, 6 This massive difference in survival reflects the so-called formula of survival, “science+education+implementation=survival”7: survival from cardiac arrest will only increase by improving our scientific understanding of the cardiac arrest-resuscitation complex, by improving development and training of evidence based guidelines, and by improving implementation of these guidelines in all steps of the clinical practice of emergency cardiovascular care (ECC).
THE PROCESS TOWARDS THE EUROPEAN RESUSCITATION COUNCIL GUIDELINES 2010The updated European Resuscitation Council (ERC) Guidelines for Resuscitation 20108 were published on October 10, 2010, and were formally presented at the 10th Scientific ERC Congress in December 2010 in Porto, Portugal. This 2010 update continues the tradition of adjusting the guidelines in a 5-year cycle.
Like in 2005, the 2010 guidelines are based on a systematic review of the most recent scientific knowledge. The International Liaison Committee on Resuscitation (ILCOR), which includes all resuscitation councils of Europe, United States, Canada, Latin America, Southern Africa, Asia, Australia and New Zealand, conducted this evidence evaluating process. The conclusions of this systematic review of the literature were presented at the Consensus on Science Conference in February 2010 in Dallas, Texas, United States, and were published as “International Consensus on Cardiopulmonary Resuscitation Science and Treatment Recommendations” (CoSTR) on October 10, 2010, in the journals Resuscitation and Circulation.9
This process illustrates the dynamic character of the guidelines-developing mechanism as used by the international resuscitation scientific community: shortly after the publication of the 2005 CoSTR and the 2005 guidelines, it became evident that our knowledge of resuscitation science is very limited and frequently extrapolated, that the evidence is scarce, and that modifications are to be guided by both scientific and educational arguments.
The principal areas of lack of scientific knowledge were identified by ILCOR in the 2007 publication by Gazmuri et al.10 This publication served as guidance for initiating new relevant research and for structuring the 2010 CoSTR and guidelines process. This 2010 CoSTR science review was conducted following a strictly standardized methodology. A total of 313 experts reviewed 277 topics. Each review was made by a team of at least 2 experts from different ILCOR member organizations. A strict “conflict of interest” policy was consequently applied in order to guarantee the best objectivity.
After each modification in the guidelines, a complex mechanism must be initiated to spread and implement the changes into clinical practice. This is a major effort that needs to be implemented by the ILCOR member organizations, by each national resuscitation council, by the thousands of instructors. We need to bear in mind that at least 2 years are needed to have new guidelines implemented in clinical practice of emergency medical services (EMS).11
Therefore, in the process of producing the 2010 guidelines the principle was applied that no changes are made unless there is sufficient new evidence, and that simplicity is crucial for a widespread acceptance worldwide.
From a scientific point of view a continuous update process with regular position papers as new science becomes available could be a reasonable option. However, in the interest of acceptance, education, and implementation it was felt important to produce an update from time to time so that this can serve as a reference for the resuscitation community. Therefore, the 5-year cycle is maintained.
GUIDELINES 2010 FOR CARDIOPULMONARY RESUSCITATION IN ADULT OUT-OF-HOSPITAL CARDIAC ARREST: WHAT HAS CHANGED AND WHY?In all aspects of the 2010 guidelines, the importance of providing good quality uninterrupted chest compressions is emphasized. Good quality chest compressions are given at a rate of at least 100 per minute with a compression depth of at least 5cm and are interrupted as briefly as possible for other maneuvers such as defibrillation, airway management, and drug administration.
The most relevant evidence-based differences with the 2005 guidelines for the basic life support (BLS) and advanced life support (ALS) management of an adult cardiac arrest victim include the following8:
Changes in Basic Life Support in the 2010 Guidelines Include 12• Early recognition with focus on unresponsiveness and the quality of breathing. The importance of gasping as sign of cardiac arrest is emphasized.
• All rescuers, trained or not, should provide chest compressions to victims of cardiac arrest. Delivering high quality chest compressions is essential. The aim should be to push to a depth of at least 5cm at a rate of at least 100 compressions per minute, to allow full chest recoil, and to minimize interruptions in chest compressions. Trained rescuers should also provide ventilations with a compression–ventilation ratio of 30:2. Telephone-guided chest-compression-only cardiopulmonary resuscitation (CPR) is encouraged for untrained rescuers.
• Minimize the duration of the pre-shock and post-shock pauses; continue compressions during charging of the defibrillator and immediately resume chest compressions following defibrillation; the delivery of defibrillation should be achieved with an interruption in chest compressions of no more than 5s.
• Routine delivery of a pre-specified period of CPR (e.g., 2 or 3min) before rhythm analysis and shock is no longer recommended.
• Further development of automated external defibrillator (AED) programs is encouraged and there is a need for wider deployment of AEDs in both public and residential areas.
• Importance of minimally interrupted high-quality chest compressions throughout any ALS intervention: chest compressions are paused briefly only to enable specific interventions.
• The role of the precordial thump is de-emphasized.
• If intravenous access cannot be achieved, drugs should be given by the intraosseous route and not via a tracheal tube.
• When treating VF/VT cardiac arrest, adrenaline 1mg is given after the third shock and then every 3-5min. Amiodarone 300mg is also given after the third shock.
• Atropine is no longer recommended for routine use in asystole or pulseless electrical activity.
• Early tracheal intubation is only recommended for highly skilled rescuers with minimal interruption of chest compressions.
• Increased emphasis on the use of capnography and on the potential harm by hyperoxemia after return of spontaneous circulation (ROSC): inspired oxygen should be titrated to an arterial oxygen saturation of 94%-98% (or 88%-92% if the patient is at risk of hypercapnic respiratory failure).
• A comprehensive, structured post resuscitation treatment protocol includes percutaneous coronary intervention (PCI), control of glycemia and seizures, and therapeutic hypothermia.
These changes are supported by good scientific evidence and are important to improve chances for survival. Changes are, therefore, based on newly available scientific evidence and by the educational need for simplification.
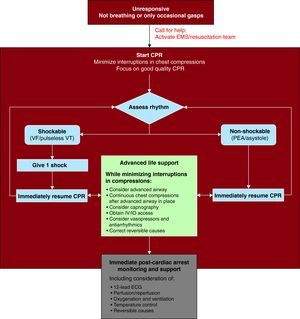
The 2010 universal algorithm illustrates the changes for CPR as agreed by all ILCOR member organizations (Figure 1).
Figure 1. The 2010 ILCOR Universal Algorithm for Resuscitation 9 (with permission from ILCOR). CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; EMS, emergency medical services; ILCOR, International Liaison Committee on Resuscitation; IO, intraosseous; IV, intravenous; PEA, pulseless electrical activity: VF, ventricular fibrillation; VT, ventricular tachycardia.
We could make the reflection: “If the science is the same why don’t we teach the same?” We know that clinical practice is guided by scientific evidence and is fine-tuned by personal experience and by patient-related, system-related, and demographic characteristics. Therefore, based on the common science, each of the major ILCOR member organizations adjusted its own guidelines for clinical practice to reflect the differences in organization, legislation, availability of drugs and devices, priorities, demographics, and characteristics of patients and rescuers.15
TOWARDS 2015: WHAT ARE THE GAPS IN OUR KNOWLEDGE?Like in 2005, it became clear already during the redaction of the 2010 guidelines that our understanding of the cardiac arrest-resuscitation complex remains very fragmentary. Progress in survival after cardiac arrest will remain poor as long as the major gaps in our knowledge are not better understood.
Progress in our scientific understanding is encouraging but remains far too slow. This is illustrated by the striking observation by Ornato et al. that the National Heart, Lung, and Blood Institute provided in the period 1985-2009 funding for 6886 studies in the area of acute myocardial infarction, 4403 in stroke, 9919 in heart failure, but only 257 in cardiac arrest and resuscitation.16 This is in sharp contrast with the knowledge that 157 000 people die from acute infarction, 150 000 from stroke, 284 000 from heart failure, but 310 000 from cardiac arrest in the United States each year. This makes clear that not only a change in the policy of the agencies and organizations that are responsible for funding translational research is required but also a multiplied interest and motivation for conducting good quality research by the investigators of the ILCOR member organizations.
Relevant gaps in our knowledge after the 2010 guidelines for the management of an adult cardiac arrest victim with BLS and ALS include the following:
• How can early recognition of cardiac arrest be optimized?
• What is the optimal strategy for preventing cardiac arrest, in and out of hospital?
• What is the optimal rate and depth of chest compression?
• What is the effectiveness of ventilation by bystanders?
• What is the optimal strategy for early access to the cardiac arrest patient at home?
• What is the best timing of chest compression after defibrillation?
• What is the effect of compression-only on survival and willingness to perform CPR?
• What is the effect of automated chest compression devices on outcome?
• When and how should bag-valve-mask be used and when should an advanced airway be best placed?
• What is the best method for confirming correct placement of a tracheal tube?
• How much oxygen is needed depending on clinical conditions, and how should it be delivered?
• What is the optimal ventilation rate and tidal volume?
• What is the role of pacing in CPR?
• What is the effectiveness of drugs (analgesics, sedatives, atropine, amiodarone, vasopressors, buffers, calcium, antithrombotics, antiplatelets, fluids, etc.) for survival in different clinical conditions?
• Are there simple reliable variables for prognostication and for starting/discontinuing treatment?
• What is the role of PCI after ROSC, and how should we treat cardiac arrest during PCI and after cardiac surgery?
• What is the optimal strategy for early reperfusion in acute coronary syndromes?
• What is the optimal temperature, timing, method, speed for therapeutic hypothermia, during and after cardiac arrest?
• How should we adjust organ donor protocols to the new treatment strategies (including therapeutic hypothermia)?
Any type of quality management in medicine, as in industry, is based on the paradigm that we need to know our current position and we need to know our final objectives so that a plan towards these objectives can be made.
Therefore, reliable baseline data are essential: reliable baseline data about epidemiology of cardiac arrest, about the process of cardiac arrest and resuscitation, about outcome. And this is exactly what is missing. Not only in Europe but also worldwide.
The international resuscitation community, ILCOR, has developed the Utstein nomenclature, a common language and set of definitions to describe the cardiac-arrest-resuscitation complex. This uniform language is essential for understanding the nature of reported results and comparing data from different origins. The Utstein nomenclature was developed in 1991 for out-of-hospital and in-hospital cardiac arrest17, 18 and was updated in 2004.19 Now, 7 years and 2 guidelines later, it might be appropriate to consider a new update of the original template and to adjust it to the modern practice and guidelines including changing characteristics of cardiac arrest victims in private and public places, simplified BLS and AED by lay rescuers, new ALS protocols, new knowledge about drugs and devices, therapeutic hypothermia, PCI, etcetera.
And we should always remember that reported data have to be interpreted in the context of inclusion and exclusion criteria:
• Are all cardiac arrest events included? Only EMS attended cardiac arrest? Only with presumed cardiac origin? Adults and children, out of hospital, at home, in hospital?
• Are patients not considered for resuscitation and why?
• Are the data from voluntarily reporting centers or from a systematic report in the complete region/country?
As a result, simple questions remain unanswered so far:
• What is the incidence of cardiac arrest in Europe?
• What is the outcome after cardiac arrest in Europe?
• How can we learn from practice in regions with the best results to improve our individual local chain of survival?
Europe could be defined according to the Council of Europe20 and includes 47 countries with a total 830 million population. The ERC has decided to adopt this definition, which is somewhat different for other regional or political definitions. This is most important, as we know that each of these 47 countries has its own demographics, health priorities, organization, system of care, legislation, and tradition.
Several investigators have attempted to estimate the incidence of cardiac arrest in Europe and also in North America. These reports are most instructive, but they share the same limitation that they report on data from selected motivated centers that may not reflect real life. Landmark articles were published by Sans et al.21 who looked into the mortality data in 30 European countries, Herlitz et al.22 who compared data from 38 European EMS systems, Atwood et al.23 who collected data from 37 European centers in 1980-2004, and Berdowski et al.6 who analyzed reports from 30 European EMS centers in 1986-2009. Many other reports were published in the context of specific questions (such as the report by Böttiger et al.24 on fibrinolytics in cardiac arrest, the Euro Heart Surveys on the use of PCI25, 26 and the cardiovascular statistical reports of the European Commission27).
These reports have also the same limitations that they cover only parts of Europe, over a long time period, data may have a reporting bias, only EMS attended cardiac arrests are included with limited information about in-hospital and at-home events, and finally they provide limited information about the complete sequence of “input-process-output” or “patient-intervention-outcome.”
Similarly, in North America several multicenter registries are in place, with the same objectives of monitoring, analyzing, and improving the sequential links of the chain of cardiac arrest and resuscitation. Three registries in high-quality sites, the Resuscitation Outcomes Consortium (ROC) Epistry Cardiac Arrest.28 the Cardiac Arrest Registry to Enhance Survival (CARES)29 and the American Heart Association's National Registry of CardioPulmonary Resuscitation (NRCPR),30 benefit from a standardized EMS system and a uniform legislation that allows valid comparison between sites.
The situation in Europe is fundamentally different from that in North America: all individual countries have EMS systems of varying types and legislation, EMS is organized either as a single or as a two-tiered system, ALS may be performed by paramedics, nurses or physicians; all of this makes a unified registry in Europe a more complex issue.
As a result, our best guess is that in Europe there is an incidence of cardiac arrest of all causes of 0.4-0.9/1000/year with a survival ranging between 6% and 31%. For cardiac arrest presenting in VF/VT we assume that the incidence is 0.2/1000/year with a survival ranging between 8% and 43%.
The reasons for these observed differences in survival are unclear: are they facts or artifacts? In this population of 830 million in 47 countries, with the wide variability of demography, legislation and systems of care, the observed differences may be a result of differences in definitions, in inclusions, in data collection systems, in data quality, or the differences may be real.
In 2008, the ERC set up a working group with the objective of creating a uniform European Registry of Cardiac Arrests (EuReCa), based on the existing experiences from member countries and on the uniform definitions of the Utstein nomenclature. It must encompass variations in EMS structure, organization, and interventions, while including the involvement of diverse participants including bystanders, ambulance personnel, and critical care specialists.
Collecting the complete core Utstein data set may be challenging and may be a difficult-to-reach objective for EMS organizations. This should, however, not discourage EMS systems to monitor their performance. We need to be aware that different parts of the Utstein core data set are needed for understanding the epidemiology of cardiac arrest, the process of resuscitation, and the eventual outcome. EMS systems may initially manage to register specific parts of the data set but not all.
The primary purpose of the ERC is to improve the quality and outcome of resuscitation applied to cardiac arrest victims in Europe. EuReCa can facilitate that goal because it will permit:
• Standardization of definitions so that they may be more uniformly applied.
• Valid comparison of process and outcome between regions and countries.
• Identification of weaknesses of local, regional, or national links in the chain of survival, and assistance in improvement.
• Monitoring the implementation and the effect of new guidelines.
• Creation of a network for national and international scientific cooperation in the field of CPR.
As a first activity of EuReCa, data of cardiac arrest treated were collected in 2008 by the EMS in 5 different regions in 5 European countries: Belgium, Germany, Netherlands, Sweden, and Spain, representing 34.9 million population and reporting on 12446 attempted resuscitations in one year. The incidence of cardiac arrest varied between the regions from 17 to 53/100000 inhabitants/year and the rate of admission to hospital from 5 to 18/100000 inhabitants/year.
This first data collection illustrated the limitations and obstacles for nationwide data collection, including legal obstacles for communicating data about the pre-hospital and in-hospital scene and about survival. It confirmed also the previously observed 3-fold difference in incidence, bystander involvement, AED use, and survival: facts or artifacts?
In summary, all European resuscitation organizations and national and European authorities have the shared responsibility to monitor the massive burden of cardiac arrest in the community, to implement the current evidence-based guidelines, to report and to improve the resuscitation process and outcome within their regions. This is possible only with registration of key variables and using a uniform style of reporting that can permit identification and consequences of differences in the systems of care within Europe. At the level of the European Union, there is now a growing awareness of the impact of cardiac arrest, of the need for access to reliable data on cardiac arrest and outcome, so that a dynamic policy for implementing optimal treatment of cardiac arrest can be supported in Europe.
Conflicts of interestNone declared.
Corresponding author: University of Antwerp, Faculty of Medicine, Universiteitsplein 1, BE2610 Antwerp, Belgium. leo.bossaert@ua.ac.be