In the western world, the prevalence of mitral regurgitation—particularly that due to degenerative disease—has gradually increased despite a substantial decrease in rheumatic disease. If present, secondary ventricular dysfunction, potentially irreversible when clinically diagnosed, requires close echocardiographic follow-up in order to establish a subclinical diagnosis. Thus, echocardiography has become an essential tool in managing patients with mitral valve regurgitation. As well as assessing parameters of ventricular geometry, in the hands of an expert echocardiography offers systematic documentation of lesion in each segment, which together with the dysfunction type should give an accurate idea of the complexity involved in the valve repair. This is increasingly relevant given the growing number of asymptomatic patients referred for mitral valve surgery. Consequently, the echocardiographic study performed prior to referral is crucial to successful mitral valve repair and cardiologists, cardiac imaging experts, and surgeons should be guided by results when referring patients to specialists with the skills necessary to undertake adequate repair of the lesions found.
Keywords
.
INTRODUCTIONThe prevalence of mitral regurgitation (MR) in the western world is on the increase1 despite the substantial reduction in rheumatic disease.2 Chronic organic MR (particularly when degenerative in origin) is the most common valvular disease: moderate or severe MR is found in 1.7% of the general population, 6.4% of patients aged 65-74 years, and 9.3% of those >75 years.3.
In the field of mitral valve repair surgery, Alain Carpentier radically changed the prognosis and clinical management of patients with MR.4 Since then, 2-dimensional, Doppler, and 3-dimensional echocardiography have gained much importance because they reveal the functional anatomy of the mitral valve (MV) and its dynamic structure. Today, an exhaustive echocardiographic study should always be the starting point when planning treatment of patients with MR. Echocardiography should provide precise data on the type and extent of valve lesions, the regurgitation mechanism, and its etiology, severity, and finally—the main objective of the present study–probability of repair.5.
SURGICAL ANATOMY OF THE MITRAL VALVEThe human MV is a complex 3-dimensional mechanism made of independent elements that constitute an anatomically dynamic structure.6 Functionally, it entails perfectly coordinated interaction between the valves or leaflets, mitral annulus, subvalvular apparatus (chordae tendineae and papillary muscles), and the left ventricle.
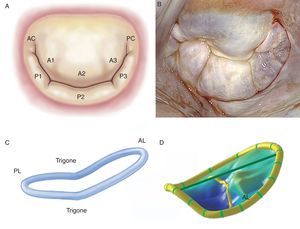
The Leaflets and CommissuresThe MV has 2 leaflets, labeled anterior and posterior. They have similar surfaces and thickness (∼1mm), are separated by their respective commissures, and are anchored at the base to fibromuscular tissue of the annulus and at the free borders to the subvalvular apparatus by means of the chordae tendineae. Achieving an optimal coaptation line requires perfect adaptation between the free surfaces of the leaflets and the native MV orifice. The anterior leaflet is trapezoidal in shape, extends vertically, and is anchored to one third of the annular circumference. Thanks to its intimate relation with the aortomitral curtain, it is a continuation of the left ventricular outflow tract. The posterior leaflet is transversal to the MV orifice and, together with the commissures, is fixed to two thirds of the annular circumference. The posterior leaflet is closely related to the left ventricle wall base, the point of greatest systolic stress. Moreover, MVs have 2 clearly distinguished zones between the base and the free border: the atrial or membranous zone (smooth and translucent) and the coaptation zone (rough, nodular, and much thicker due to the fusion of numerous chordae tendineae). In histopathologic terms, we can discern 3 separate layers: the fibrous layer (a continuation of the chordae tendineae), the spongiosa (organized collagen fibers, proteoglycans, elastin, and other connective cells) and the fibroelastic layer, covering most of the leaflet surface (elastin and collagen). Moreover, as a surgical reference, the MV can be subdivided into 8 segments if the commissures are counted individually (Figure 1).7 Unlike the anterior leaflet, the posterior leaflet has 2 clefts in its free border that permit it to open fully during diastole or ventricular filling and which, in turn, separate the 3 segments. Of these, the middle one has much more redundancy and its thickness varies with the impact of greater systolic pressure, which would explain why it is more prone to prolapse and lesions.8, 9.
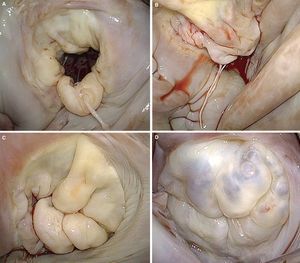
Figure 1. A, drawing of Carpentier's classification (adapted from Carpentier et al. 7 by permission of the author). B, surgical view of the mitral valve with signs of degenerative disease, seen from the left atrium. C, three-dimensional reconstruction of the saddle-shaped mitral annulus; the highest point corresponds to the anterior portion and the lowest to the trigones. D, three-dimensional reconstruction of mitral leaflets together with mitral annulus, showing anterior leaflet prolapse. AC, anterior commissure; AL, anterior leaflet; PC, posterior commissure; PL, posterior leaflet.
The commissures are triangular segments that establish continuity between the 2 leaflets. To identify them, we use the vertical axis of the papillary muscles and their corresponding chordae tendineae as a reference and thus obtain an anterior and posterior commissure.
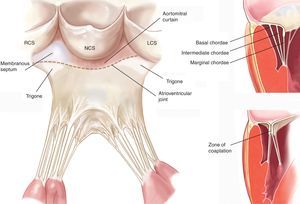
The Mitral AnnulusThe anatomic link between the left atrium and the left ventricle resembles a hinge of fibrous tissue and it is here that MV motion begins. This structure, labeled the mitral annulus, is an integral part of the fibrous skeleton of the human heart. The part of the annular zone where the posterior leaflet is inserted is 2mm from the fibrous tissue hinge and consists of a very thin band of connective tissue. As this segment of the annulus is not connected to any rigid structure, annular dilatation and calcification occur here in a great many patients.10 In contrast, the zone where the anterior leaflet is inserted is practically a continuation of the aortomitral curtain reinforced at the base by 2 rigid structures. These are both fibrous trigones: the right fibrous trigone (joining the membranous septum, mitral annulus, tricuspid annulus, and noncoronary cusp of the aortic annulus) and the left fibrous trigone, contiguous to the left coronary cusps of the aortic annulus and the left border of the mitral annulus. The normal mitral annulus is more or less elliptical (D-shaped) and shows greater eccentricity (is less circular) in systole than in diastole.11 Moreover, it presents a 3-dimensional saddle-shaped configuration with 2 lower points (trigones) and 1 peak at the midpoint of the anterior leaflet (Figure 1). This is always above the midpoint of the posterior leaflet. Annular area is 5cm2 to 11cm2 (mean, 7cm2) and is modified during the cardiac cycle. It increases in size at end-systole, continues to do so during isovolumic relaxation and reaches its maximum at end-diastole.12 Reduction in annular size begins with atrial contraction and reaches its maximum halfway through the systolic cycle, leading to optimal coaptation of both leaflets. It is worth noting that the changes in the annular surface occur practically at the will of the posterior annulus, as the anterior part of the annulus is virtually immobile. Finally, the mitral annulus also presents an oscillating or vertical movement towards the left atrium during diastole and towards the ventricular apex during systole. Normal annular contraction is estimated at 25%.13.
The Chordae TendineaeThe chordae tendineae are filament-like structures of connective fibrous tissue that join the ventricular surface and free border of the leaflets to the papillary muscles and, by default, the left ventricle posterior wall. Approximately 25 primary chordae begin in the papillary muscles and progressively subdivide to insert themselves into the leaflets. They are classified according to the point of insertion between the free border and the leaflet base. Marginal chordae are inserted in the free border of the leaflets and their function is to avoid leaflet prolapse. The intermediate or second-order chordae are inserted in the ventricular face of the leaflets and their principal function is to relieve excess tension in valve tissue. Basal or third order chordae are only found on the posterior leaflet and connect its base and the posterior mitral annulus to the papillary muscles (Figure 2).
Figure 2. Drawing of mitral subvalvular apparatus (left panel adapted from Carpentier et al. 7 by permission of the author). LCS, left coronary sinus; NCS, noncoronary sinus; RCS, right coronary sinus.
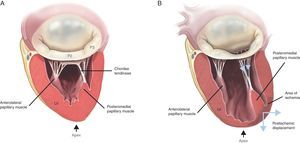
Papillary MusclesTwo organized groups of papillary muscles exist. They are labeled with reference to their position in relation to the mitral commissures. The anterolateral papillary muscle has a single body, is larger, and is irrigated by the first obtuse marginal branch of the circumflex artery and the first diagonal branch of the anterior descending artery. The posteromedial papillary muscle has 2 bodies, is smaller in size, and is irrigated only by the posterior descending artery, a branch of the right descending coronary artery, in 90% of cases and by the circumflex artery in the other 10%.14 Hence, the posteromedial muscle is always much more vulnerable to ischemic episodes.
The Left VentricleThe continuum from the papillary muscles to the left ventricle gives the latter a dominant role in mitral leaflet motion control, particularly in patients with ischemic disease. According to Starling's law, myocyte contractility would compensate for excess volume in the presence of MR, especially in its early stages.15 However, given that the left ventricle actively sustains all the mitral apparatus, any amount of pathologic dilatation—whether ischemic in origin or not—would lead to functional MR.16.
THE PATHOPHYSIOLOGIC TRIADMR is characterized by the existence of blood flow in systole from the left ventricle to the left atrium.17 Any minimal lesion can cause MR by reducing or eliminating mitral leaflet coaptation during systole. Hence, the study (localization and magnitude) and subsequent precise description of mitral lesions are essential to determine the chances of successful valve repair and proceed with an appropriate therapeutic plan, tailored to each individual patient.18 Carpentier established the “pathophysiologic triad” of MR to manage this particular group of patients in an ordered, systematic way.19 The triad highlights the importance of distinguishing between the condition causing MR (etiology), the resulting lesions, and finally how these lesions affect leaflet motion–ie, the type of dysfunction caused. With the passage of time, cardiovascular specialists have adopted this triad classification and today, although not yet in general use, it promotes clear mutual understanding between surgeons and cardiac imaging specialists.
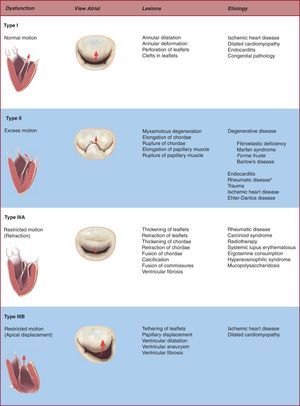
Valve DysfunctionIt is vitally important that we identify the etiology and lesions that lead to clinical MR because choice of therapy and long-term results can differ substantially as a function of clinical context. It is also crucial to describe the valve dysfunction secondary to these lesions or the mechanism underlying MR, particularly when trying to establish the chances of successful repair. The classification of mitral dysfunction is based on the position of the leaflet margins with respect to the mitral annulus plane: a) type I dysfunction, normal leaflet motion with severe annular dilatation (central regurgitant jet) or perforation of one of the leaflets; b) type II dysfunction, excessive leaflet motion generally secondary to pathologic elongation or rupture of the chordae tendineae (in which case regurgitant jet is directed to the opposite side of the affected leaflet), and c) type III dysfunction, restricted leaflet motion due to retraction of the subvalvular apparatus (IIIa, frequent in rheumatic disease or inflammatory processes) or papillary muscle displacement (ischemic remodeling or dilated cardiomyopathy) causing apical displacement (tethering) of the valve (IIIb). Regurgitant jet is directed at the same side as the valve affected.
Etiologies and LesionsWorldwide, rheumatic diseases are still the most frequent cause of MR but they have ceased to be its most common cause in the developed countries. Ischemic disease, currently responsible for 20% of MR, could soon lose importance thanks to the ever more aggressive treatment of coronary disease. However, degenerative disease is today the most frequent cause of MR.20.
Degenerative disease of the MV presents a spectrum of lesions21 from chordal rupture with prolapse of a single segment of an otherwise totally normal valve to multiple segment prolapse in both valves, accompanied by excess tissue and marked annular dilatation.22, 23 Moreover, the range of degenerative disease lesions gives rise to the distinction between 2 opposing clinical entities: fibroelastic deficiency (FD) and Barlow's disease (BD). All the etiologies and lesions implied in MR development are shown in Figure 3.
Figure 3. Pathophysiologic triad of mitral regurgitation. *Finding leaflet prolapse in the context of rheumatic disease only occurs if a type III dysfunction exists or pseudo-prolapse is identified.
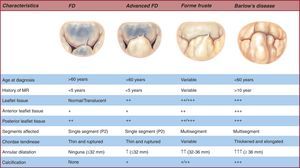
FD generally occurs in patients aged >60 years with a relatively short history of valvular disease and severe holosystolic MR.24 The term “fibroelastic” describes a pathologic condition associated with a deficit of the protein fibrillin25 that often leads to progressive weakening26 and elongation and rupture of the chordae tendineae,27 usually involving the posterior leaflet mid-segment.28 Chordal rupture is the most frequent lesion in FD.29 The leaflets are thin and translucent, although occasionally the prolapsing segment may present myxomatous characteristics and distension if disease has been present for a long time.30 The key to distinguishing FD from other entities is exhaustive analysis of segments contiguous to the one that has prolapsed.31 In this condition, the segments are usually totally normal, with no change in height, size, or tissue properties.32 Finally, annulus size,33 defined by anterior valve surface, is generally <32mm (Figure 4).
Figure 4. Spectrum of degenerative disease of the mitral valve. FD, fibroelastic deficiency; MR, mitral regurgitation.
At the opposite end of the spectrum of degenerative diseases we find BD,34 primarily found in young patients, generally aged <60 years, when referred for surgery. These patients present a long history of cardiologic follow-up35, 36 for cardiac murmurs.37 In this context, the leaflets present much more diffuse, complex lesions. We often find prolapse and myxomatous degeneration38, 39 of many segments in one or both leaflets (Figure 4, Figure 5). The most common lesions are excess tissue and therefore leaflet thickening and distension, as well as elongation, thickening, and/or the rupture of many chordae tendineae.40 In these patients, annulus size is ≥36mm.41 Finally, it is not infrequent to find several degrees of calcification both in the annulus and subvalvular apparatus, in particular on the posterior face of the annulus and the anteromedial papillary muscle.42.
Figure 5. Surgical images of the lesions most frequently associated with degenerative disease of the mitral valve. A, posterior prolapse due to chordae tendineae rupture. B, anterior prolapse due to elongation, thinning and rupture of chordae tendineae. C, anterior and posterior prolapse secondary to elongation of the chordae tendineae and myxomatous degeneration of several segments; note the pathologic clefts in the posterior leaflet. D, Barlow's disease with myxomatous degeneration of both leaflets.
Ischemic MR can present acutely with papillary muscle rupture43 (organic ischemic MR) or as a consequence of left ventricular remodeling and apical and inferior displacement of the papillary muscles44 (functional ischemic MR), which pull the mitral leaflets and cause tethering of the leaflets and of their point of coaptation45 (Figure 6). Identifying this type of lesion in the echocardiographic study is of the greatest importance given that long-term survival following ischemic MR, even with mild ischemic lesions, is notably limited.46 When restricted leaflet movement occurs principally in systole,47 it gives rise to an asymmetric restrictive pattern, mainly observed in patients with posterior infarction and posterior leaflet restriction.48 In contrast, in patients with dilated cardiomyopathy or anterior and posterior infarctions, both leaflets present a restrictive deficit giving rise to a symmetric pattern49 (Figure 7).
Figure 6. Mechanism of functional mitral regurgitation. A, normal mitral valve. B, ischemic mitral valve with pronounced posterior restriction in P3 after an episode of ventricular ischemia. LV, left ventricle.
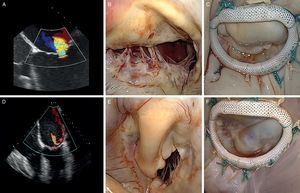
Figure 7. Echocardiographic evaluation and surgical images of ischemic mitral regurgitation (A-C, symmetrical pattern; D-F, asymmetrical pattern). A, central regurgitant jet. B, symmetric restriction. C, note the symmetrical line of coaptation following repair. D, posterior regurgitant jet. E, asymmetric restriction. F, note the angle of the line of coaptation following annular repair.
ECHOCARDIOGRAPHIC EVALUATIONWhen conducted by an expert, systematic examination of the MV using transthoracic echocardiography (TTE) should reliably predict repair.50 However, more and more centers now opt for transesophageal echocardiography (TEE), particularly in complex cases, prior to referring patients for surgical evaluation.51 An echocardiographic representation of the MV should provide information that is both general52 (previous illness, posterior or bilateral) and specific to leaflet segments (individual analysis of each segment); it should identify an excess or scarcity of tissue in the leaflets (etiologic differentiation),53 evaluate annular dimensions, detail subvalvular apparatus status, and estimate ventricular resistance.54 To do so, the systematic examination should include 4 transesophageal projections (4-chamber, commissure, 2-chamber and long-axis), as well as a gastric projection (short-axis) (Figure 8).
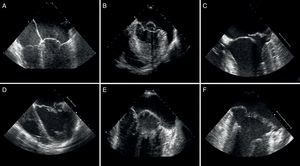
Figure 8. Echocardiographic distinction between fibroelastic deficiency (A-C) and Barlow's disease (D-F). A-C, note the presence of fine nonmyxomatous leaflets, only one affected segment and prolapse generally due to chordae tendineae rupture. C-E, thickened leaflets with marked myxomatous degeneration of several segments or both leaflets affected and atrial displacement of the posterior leaflet fibrous tissue hinge.
Four-Chamber ViewIf we use TTE, the classic apical 4-chamber view will enable us to analyze the anterior leaflet, especially A2 and A3, as well as the lateral segment of the P1 posterior leaflet. In contrast, TEE enables us to observe practically all segments as a function of the degree of rotation of the transducer. At 0°, the transducer shows both leaflets’ mid-segments (A2 and P2). If the probe rotates 20°, it cuts the line of coaptation obliquely and obtains detailed information for the most lateral segments, such as A2, A1, and P1.
View of Both CommissuresThis can be obtained with an apical 2-chamber plane in TTE or by rotating the transesophageal probe approximately 60° so that the image plane cuts perpendicularly through that which delimits both commissures, thus cutting through both valves to facilitate analysis of P3 (to the left of the image), A2 (in the center of the image), and P1 (to the right of the image). In this plane, the papillary muscles can normally be seen. Moreover, this view is of great value in determining which segment is pathologic because if the regurgitant jet begins to the left of the line of coaptation, we deduce it is secondary to a lesion in P3 or A3; if the regurgitant jet starts to the right of the line of coaptation, the segments involved will be P1 or A1. The height of P1 and P3 can be calculated from this plane, which is highly important when predicting repair complexity. If these segments are >1.5cm in height, we can assume anterior systolic movement is more likely following repair; consequently, under these circumstances, the repair will be more complex as it requires partial resectioning of the leaflets.55.
Two-Chamber ViewIf we continue rotating the transducer to 90°, we obtain a 2-chamber view. In this plane, P3 can be seen to the left of the image and all the anterior leaflet segments are located to the right. This view is crucial to analysis of the anterior leaflet and complete evaluation of the posteromedial part of the line of coaptation (A3 and P3), as well as its corresponding commissure.
Parasternal or Sagittal Long-Axis ViewThe long axis of the parasternal plane, using TTE, or the sagittal view with the probe rotated to 120°, using TEE, will cut the line of coaptation perpendicularly, through P2 (to the left of the image) and A2 (to the right of the image). This view is especially important as prolapse of P2 is the most frequent, particularly in patients with degenerative disease. Moreover, this view enables us to evaluate the annular surface, extrapolating annular diameter from the anterior leaflet surface. Pathologic annular dilatation is considered present when the annulus:anterior leaflet ratio is >1.3 or annular diameter is >35mm. Moreover, the surgeon should know of any substantial calcification in the annulus or subvalvular apparatus, as surgical strategy changes radically when facing calcified segments or extremely rigid tissue.
Parasternal Short-Axis or Transgastric ViewThis view, although requiring an experienced operator, can be obtained by TTE (classic parasternal view) or TEE (0° transgastric view). In diastole, it enables us to evaluate all segments and both commissures. In systole, we can deduce the localization of the pathologic segment by analyzing regurgitant jet. Moreover, we can obtain information about the subvalvular apparatus and the distance between the head of the papillary muscle and mitral annulus.
THREE-DIMENSIONAL ECHOCARDIOGRAPHYThe latest technological advances in 3-dimensional echocardiography have enabled us to obtain real-time images of the MV and much greater detail, which significantly adds to our knowledge of MV anatomy and functioning.56 This is particularly the case in geometric evaluation of the mitral annulus.57 It has been shown that the mitral annulus is saddle-shaped.58 The highest points, ie, those furthest from the cardiac apex, correspond to the anterior region closest to the aortic root and the posterior region closest to the left ventricle posterior wall.59 In contrast, the lowest points correspond to the mitral commissures. In healthy individuals, the saddle shape is more marked in mid-systole, when annular area is at its minimum. At the end of both phases, the annulus presents a much flatter, more extended shape.60 These variables gain greater clinical interest when we analyze the anatomy of patients with functional MR. In this context, we can observe marked dilatation of the intercommissural diameter and anteroposterior diameter, because of which the annulus takes on a flattened, circular shape.61 If the infarction is anterior, the annulus becomes flatter than if it is inferior.
Three-dimensional echocardiographic analysis of the mitral leaflets (together with the annulus) provides the surgeon with the most important data. Currently, we can trace the exact location of the mitral leaflets and measure their area using special computer software. Due to the saddle shape of the annulus, 2-dimensional echocardiography can overestimate the existence of prolapse due to apparent leaflet displacement towards the left atrium. During 3-dimensional reconstruction, the prolapsing segment becomes convex when viewed from the atrium, and concave when viewed from the ventricle. Moreover, this correlates exactly with the anatomic examination the surgeon conducts during the intervention (superior orientation of the anterior leaflet and inferior orientation of the posterior leaflet), with >96% localization of the prolapse.62 Three-dimensional echocardiography can also locate all the points of surgical interest: a) annular area and possible size of the mitral ring that will be implanted; b) leaflet surface; c) prolapsed segment and volume of prolapse; d) tenting volume (volume between the annulus and the mitral leaflets); e) tethering distance (between any point on the mitral annulus and the papillary muscles), and f) interpapillary distance63 (Figure 9).
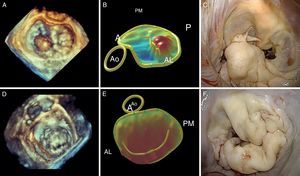
Figure 9. Correlation of conventional preoperative echocardiography and 3-dimensional echocardiography with intraoperative images. A, anterior; AL, anterolateral; Ao, aorta; P, posterior; PM, posteromedial.
MITRAL SURGERYThe echocardiographic study prior to patient referral is decisive in achieving effective mitral valve repair and should inform patients, cardiologists, and surgeons preparing the optimal route-map for each patient with MR.64, 65 In this context, it is increasingly important to evaluate mitral valve disease exhaustively to refer each patient to the surgeon with the skills necessary to repair each of the observed lesions.66 The systematic documentation of the disease found in each segment, together with leaflet dysfunction type, should provide a precise idea of the complexity of repairing the valve in question.67.
Several echocardiographic parameters are identified in the literature as possible predictors of failure in MV repair68 (Table 1). They include severe central regurgitation jet, major annular dilatation (≥50mm), 3 or more affected segments, anterior leaflet lesions, or intense calcification, among others.69 Moreover, the scarcity of leaflet tissue is also an important risk factor both in rheumatic disease and in patients with infective endocarditis or degenerative disease with advanced FD.70 In ischemic disease, TEE findings of ≥37mm diastolic annular diameter, ≥1.6cm2 tenting area, and severe MR can lead to mitral valve repair failure in 50% of patients during clinical follow-up.71 In contrast, with transthoracic transducer echocardiography,72 >1cm coaptation distance, >2.5cm2 systolic tenting area, >45° posterior leaflet angle (posterior leaflet restriction), central regurgitation jet (indicating substantial restriction of both leaflets), central or posteromedial regurgitant jet, and significant ventricular hypertrophy (ventricular remodeling following repair) increase the risk of repair failure.73.
Table 1. Echocardiographic Predictors of Failed Mitral Valve Repair.
| Organic MR | Functional MR |
| Severe central jet | Coaptation distance ≥1 cm |
| Annular dilatation ≥50 mm | Tenting area >2.5cm2 |
| Lesions in 3 or more segments | Posterolateral angle >45° |
| Lesions of the anterior leaflet | Interpapillary distance >20 mm |
| Severe calcification | Ventricular akinesia |
| Scarcity of tissue in the leaflets | EDD >65mm or ESD >51 mm |
| Opposite dysfunction | Sphericity index >0.7 |
EDD, end-diastolic diameter; ESD, end-systolic diameter; MR, mitral regurgitation.
The decision to operate in a patient with MR is the result of a complex process entailing the evaluation of many variables,74, 75 including MR severity, its impact on atrial and ventricular remodeling, ventricular function, pulmonary pressures, the chances of successful repair, comorbidities, operative risk, and the state of the patient's symptoms.76, 77 If we analyze each parameter individually, it would be no exaggeration to say that any variation in current treatment of severe MR depends entirely on the echocardiographic study. In fact, increasingly less importance is given to the patient's symptoms and correspondingly more importance to ventricular geometry data.78 In this respect, opinions differ as to the right time for surgery.79 The first, more traditional, widely agreed view recommends careful follow-up (echocardiography every 6 or 12 months) until the appearance of symptoms or clear indicators of ventricular dysfunction such as left ventricular ejection fraction <60% or end-systolic diameter >40mm (>45mm in European guidelines).80, 81, 82 Moreover, when ventricular function is normal, surgery is recommended in the presence of pulmonary hypertension (>50mmHg) or atrial fibrillation.83 In contrast, the second view,84 which is much more up-to-date and recommends surgical intervention in asymptomatic patients with conserved systolic function, is much more controversial.85 Thus, surgery would always be recommended in asymptomatic patients if we have echocardiographic data that confirm the severity of MR,86 analysis indicates >95% chances of repair, and operative mortality is <1%.87 These requirements presuppose the closest possible collaboration between a cardiologist experienced in echocardiographic quantification, a surgeon who performs around 50 mitral valve repair interventions a year,88 and an experienced intensive care unit89 (Table 2). Although a range of opinions and approaches to management is important, we believe the individualized treatment of each patient should not exclude any alternatives if these are appropriately employed (Figure 10). Moreover, individualized treatment should mean choosing the appropriate surgical technique90, 91, 92, 93 for each of the lesions found.
Table 2. Echocardiographic Pattern of Referral for Surgery to Optimize the Probability of Mitral Valve Repair (Approximate Stratification by Number of Cases per Year Performed by a Single Surgeon).
| Etiology | Dysfunction | Calcification | Lesions | Probability of repair | |
| <50 cases/year | ≥50 cases/year | ||||
| Fibroelastic | II | No/annular | Posterior localized prolapse (P2) | Feasible | Feasible |
| II | No/annular | Anterior prolapse | Probable | Feasible | |
| Barlow | II | No/annular | Posterior localized prolapse (P2) | Feasible | Feasible |
| II | No/annular | Prolapse of 3 or more segments | Probable | Feasible | |
| II | Leaflets | Prolapse of 3 or more segments | Improbable | Probable | |
| II | No/annular | Anterior prolapse | Improbable | Probable | |
| Endocarditis | I | No | Perforation | Probable | Feasible |
| II | No | Prolapse | Probable | Feasible | |
| II | No | Destructive lesions | Improbable | Probable | |
| Rheumatic | IIIA | Annular | Malleable anterior leaflet | Probable | Feasible |
| IIIA | Leaflets | (Rigid) calcified anterior leaflet | Improbable | Improbable | |
| (Functional) ischemic | I | No | Dilatation or annular deformation | Feasible | Feasible |
| IIIB | No | Tethering | Feasible | Feasible | |
| IIIB | No | Predictors of repair failure | Improbable | Probable | |
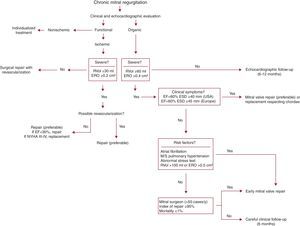
Figure 10. Proposed algorithm for treatment of mitral regurgitation. EF, ejection fraction; ERO, effective regurgitant orifice; ESD, end-systolic diameter; M, moderate; NYHA, New York Heart Association; RVol, regurgitant volume; S, severe.
When dealing with patients with ischemic MR, the risk of papillary muscle or even ventricular muscle rupture is high and surgery should be immediate. However, surgical risk is greater and although symptoms may improve following restrictive annuloplasty,94 the long-term benefit to survival has not been proven. Moreover, implantation of a restrictive ring (2 sizes smaller than measured) can cause postoperative mitral valve stenosis and not correct the underlying problem of papillary muscle displacement.95 Hence surgical studies are being conducted into posterior leaflet extension in P3 with pericardial tissue, to relieve tension on the leaflets without the need for restrictive annuloplasty. Current trends suggest that in mild or moderate ischemic MR (effective regurgitation orifice [ERO] <0.2cm2), we should consider surgical correction using annuloplasty but if ERO is >0.2cm2 (severe ischemic MR), valve replacement and myocardial revascularization can be equally feasible alternatives to mitral valve repair.
CONCLUSIONSWithout doubt, imaging studies are becoming increasingly important in the management of patients with MR, particularly given the trend for early interventions in patients with severe asymptomatic MR. Precise follow-up of changes in geometry and volume is possible and this facilitates establishing surgical indications for patients with a tendency towards ventricular dysfunction before ejection fraction declines. Three-dimensional echocardiography gives a perfect “surgeon's-eye view” of the MV, enabling preoperative identification of complex lesions that require referral to a specialized center. The incessant training of future generations of specialists is clearly crucial in avoiding unnecessary replacements. Multidisciplinary evaluation of the patient with MR should become standard practice when choosing the appropriate therapeutic strategy.
FUNDINGThis project was financed by The Mount Sinai Medical Center, New York, United States.
CONFLICTS OF INTERESTNone declared.
Corresponding author: The Mount Sinai Medical Center, 1190 Fifth Avenue GP2W, New York, NY 10029, United States. javier.castillo@mountsinai.org