Keywords
INTRODUCTION
Concept
Cerebrovascular disease (CVD) is one of the most common reasons for neurological emergencies and constitutes a serious public health problem.
Data from the World Health Organization indicate that it is the second most common cause of death and the leading cause of disability. In Spain, it is the leading cause of disease-specific mortality in women. Until 10 years ago, health care professionals and, in particular, neurologists have maintained a conservative rather than an aggressive approach to this devastating disease. Stroke units and, above all, methods for achieving reperfusion with thrombolytic therapy have made it possible to modify the approach: stroke can be a treatable entity, a circumstance that significantly improves the prognosis of these patients.
The terms CVD and stroke refer to a disturbance in the cerebral blood flow that results in a transient or permanent change in the function of 1 or more regions of the brain. There are different types of stroke, depending on the nature of the lesion. Ischemic stroke is due to a lack of blood supply to a given area of the brain parenchyma, whereas hemorrhagic stroke is caused by the rupture of a cerebral blood vessel, with extravasation of blood into the vascular bed.1 Strokes are ischemic in 85% of the cases, and the remainder are hemorrhagic.
Given the several subtypes of stroke, the variations in the profile of the disease course, the topographic features and the differences in the underlying mechanism and the etiology, there are a number of terms to describe CVD.2 It is very important to correctly identify the causative mechanism in order to choose the most appropriate treatment and apply effective secondary prevention.3
Depending on developments during the initial hours, we differentiate between 2 major types of ischemic cerebrovascular accidents: the transient ischemic attack (TIA), classically defined as a neurological deficit of less than 24 hours duration, and cerebral infarction, with irreversible damage to the brain parenchyma.
With respect to TIA, it should be pointed out that, although the term "transient" indicates a benign nature, these episodes should be considered an important warning sign of cerebral infarction or other cardiovascular complications. Approximately 15% to 30% of all cerebral infarctions are preceded by a TIA.4 Seventeen percent of the patients who present with cerebral infarction have had a TIA on the same day, 9% on the previous day and 43% during the preceding seven days.5 In addition, patients who have experienced a TIA also have a poor long-term prognosis.6 The new neuroimaging techniques have made it possible to detect an ischemic lesion, that is, a cerebral infarction, in patients whose symptoms resolved within 24 hours. These prognostic data and the better understanding of the pathophysiology of cerebral ischemia have resulted in a new temporal definition of TIA as the ischemic event that causes a neurological deficit lasting less than one hour and is not associated with cerebral infarction in neuroimaging studies.7 Today, TIA should be considered a medical emergency.
The TIA is referred to as carotid, vertebrobasilar or indeterminate, depending on the vascular territory involved, and, on the basis of the clinical signs, can be classified as retinal (amaurosis fugax), cortical hemispheric, lacunar, or atypical. Each of these TIA has its own pathophysiology, clinical features, and prognosis and, thus, the treatments differ.
In summary, patients with TIA should be considered a group at high vascular risk and, once diagnosed, the causative mechanism should be identified.8
With respect to cerebral infarction, the final cause is the lack of blood flow to some part of the brain, which produces ischemia and, ultimately, infarction (death of brain cells). The presence of ischemic brain tissue is sufficient to affect brain function and, thus, produces the typical clinical signs of stroke. The region of ischemic, but not infarcted tissue, is referred to as the ischemic penumbra, and is potentially salvageable with recanalization therapy and proper care. Today, it is possible to visualize the ischemic penumbral tissue, or mismatch, by means of neuroimaging techniques, diffusion/perfusion magnetic resonance (MR), and perfusion computed tomography (CT).
Etiological and Pathogenic Classification of Stroke
The etiological and pathogenic categorization of stroke is essential for its proper treatment. Although there is no single set of criteria, that most widely used is based on the TOAST (Trial of Org-10172 Acute Stroke Treatment)9 and SSS-TOAST10 classifications.
We can distinguish 5 etiological and pathogenic subtypes that require different treatment strategies: atherothrombotic stroke due to large vessel disease, cardioembolic stroke, lacunar stroke due to small vessel disease, that secondary to an unusual cause (arterial dissection, vasculitis, vasospasm, etc), and stroke of undetermined etiology, or cryptogenic, despite a thorough study. Likewise, we distinguish different mechanisms of stroke. Embolic stroke is the most common type, and there are 2 possible etiologies (cardioembolic and atherothrombotic due to artery-to-artery embolism originating from an atheromatous plaque), in addition to thrombosis caused by vessel wall changes, lipohyalinosis in lacunar infarction, and the hemodynamic mechanism underlying infarction in adjacent vascular territories due to hypoperfusion secondary to severe stenosis of the vascular territory involved.
The SSS-TOAST criteria are discussed below.
Criteria for Atherothrombotic Infarct (Large Vessel Atherosclerosis)
This diagnosis requires the performance of duplex Doppler and/or an angiographic study (MR angiography, CT angiography, or arteriography) to detect arterial wall lesions (stenosis and occlusion) in the large vessels, both extracranial and supraaortic, and intracranial (middle, anterior, posterior cerebral artery, or basilar trunk). Normal findings, minimal changes or the failure to carry out these studies rule out the diagnosis.
Essential Criteria
Significant stenosis (greater than 50%), occlusion, or an ulcerated plaque (over 2 mm thick) in intracranial artery or ipsilateral extracranial artery, demonstrated by duplex Doppler or angiographic study (conventional, MR angiography, or CT angiography)
Absence of cardiogenic embolism or heart disease of some other etiology
Other Criteria Indicative of this Diagnosis
1. Clinical criteria.
Presence of murmur, ipsilateral to the infarct
Presence of previous TIA, ipsilateral to the infarc
History of ischemic heart disease
History of intermittent claudication of lower limbs
2. Imaging criteria.
Presence in CT and/or MR of cortical, or subcortical nonhemorrhagic infarction measuring over 1.5 cm in carotid, or vertebrovasilar territory
Stenosis or occlusion of the involved vascular territory in angiography
Criteria for Cardioembolic Ischemic Stroke
Essential Criteria
The presence of cardiogenic embolism (see list of cardioembolic sources in Table 1)
The presence of significant cerebrovascular atheromatous lesions (see the criteria for atherothombotic infarct) and other possible etiologies must be ruled out
In the case of low-risk heart disease (see the list of cardioembolic sources), and having ruled out other causes of stroke, the condition will be classified as "possible" cardioembolic stroke.
Other Criteria Indicative of this Diagnosis
1. Clinical criteria.
Sudden maximum neurological deficit (occurring in seconds or a few minutes)
Onset during fasting
Loss of consciousness (transient) and/or seizures at onset
Multiple simultaneous cerebral infarctions
Previous cerebral infarctions or TIA in different vascular territories
- History or coexistence of systemic emboli
2. Imaging criteria.
Computed tomographic images showing infarction greater than 1.5 cm, usually cortical, sometimes hemorrhagic, or multiple infarcts in different vascular territories
Angiographic evidence of transient angiographic occlusions, isolated arterial occlusion with no evidence of atherosclerotic lesions, or central filling defect in the proximal portion of an artery with no atherosclerotic changes
Small Vessel (Lacunar) Disease
Maximum infarct diameter of 1.5 cm, located in arterial territory or perforating cerebral arterioles (the diameter of which is usually less than 200 µm) due to lipohyalinosis or microatheromatosis of said vessels
The clinical course is that of one of the classic lacunar syndromes (pure hemiparesis, pure sensory syndrome, sensorimotor syndrome, ataxic hemiparesis, or dysarthria-clumsy hand syndrome)
The presence of hypertension or diabetes mellitus supports the diagnosis
By definition, there must be no cortical signs or symptoms
There should be no potential cardiac sources of embolism or stenosis greater than 50% in ipsilateral extracranial arteries
The presence of stenosis greater than 50% or atheromatous plaques in medium-sized, or large arteries does not rule out the presence of lacunar infarction
Criteria for Infarction of Other Etiologies or Uncommon Causes
This category includes patients with acute cerebral infarction due to infrequent causes, such as nonatherosclerotic vascular diseases (inflammatory, noninflammatory, infectious, hereditary), hypercoagulability states, hematologic disorders, migraine-infarction, vasospasm, and other hereditary and metabolic diseases.
The aforementioned etiologies of cardioembolic cerebral infarction and the presence of atherosclerosis in extracranial arteries should be ruled out.
Figure 1 shows the incidence of each stroke subtype according to the Stroke Data Bank of the Spanish Neurological Society (BADISEN).
DIAGNOSIS
The rapid identification of stroke, the determination of its etiology, and pathogenesis, and its proper treatment, with specific therapies (fibrinolytic agents) during the acute phase, general care in the stroke units, and preventive treatment specific for each stroke subtype are vital in the attempt to reduce irreversible brain damage, prevent recurrence and, thus, enhance functional recovery of the patient.
The diagnostic process includes the following elements: clinical history, general physical and neurological examination, and ancillary tests.
In the clinical history, attention must be paid to the personal and family history of vascular disease, the ictal, or sudden onset of focal neurological symptoms and, above all, the time elapsed since onset in order to decide whether emergency fibrinolytic therapy is warranted.
The neurological examination should confirm the suspicion of neurological focality and will make it possible to map the topography of the stroke.
These data, together with the general physical examination, laboratory findings, the electrocardiogram (ECG), and chest -ray, will aid us in establishing the possible etiology of the stroke (carotid murmur, atrial fibrillation on ECG, cardiomegaly on chest -ray, etc.).
Among the more specific examinations, emergency cranial CT is essential to rule out cerebral hemorrhage and other causes of focal neurological signs, and to confirm the ischemic nature of the process. In the initial hours after cerebral infarction, the cranial CT may be normal or reveal early signs of infarction that will be of great use in the assessment of the extension of the infarct and initiate fibrinolytic therapy.
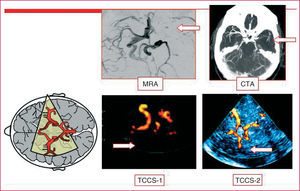
A transcranial duplex Doppler study involving supraaortic trunks will be carried out in all the patients who have had an ischemic stroke. The study of the carotid artery will enable us to diagnose underlying atherothrombotic disease and assess specific preventive treatments, such as carotid endarterectomy. Transcranial duplex Doppler enables us to diagnose intracranial stenoses, examine the collateral circulation, confirm arterial recanalization following the administration of fibrinolytic therapy (Figure 2), and even detect right-to-left shunt across a patent foramen ovale (PFO) using a microbubble solution. On the other hand, it provides valuable information in the preoperative evaluation and for the prognosis of carotid stenosis (studies of the collateral circulation and hemodynamic reserve and detection of microemboli) (Table 2). A common error is to limit the ultrasound study in patients with carotid stenosis to the examination of the neck arteries.
Figure 2. Intracranial occlusion of the middle cerebral artery. Images obtained by means of cranial magnetic resonance angiography (MRA), cranial computed tomographic angiography (CTA), and duplex transcranial color-coded sonography (TCCS 1-2).
The high sensitivity and specificity of the ultrasound study (color duplex of the supraaortic trunks plus transcranial duplex Doppler) makes it possible, by adding some other noninvasive imaging technique such as MR angiography or CT angiography, to decide whether or not to perform endarterectomy, without utilizing digital subtraction angiography.
The presence of potential cardiogenic embolism should be assessed on the basis of clinical history, physical examination, chest -ray, and ECG. This makes it possible to initiate preventive anticoagulant therapy. The performance of transthoracic echocardiography is recommended when previous examinations have detected no evidence of disease, when a cardioembolic etiology is suspected and in strokes in young patients. In those cases in which aortic atheromatosis is suspected and when a right-to-left shunt is detected, the study should be completed with transesophageal echocardiography.
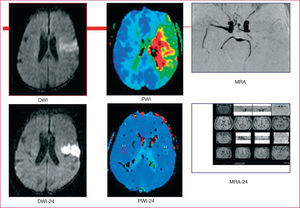
Cranial MR is highly useful in the treatment of stroke, not only because it helps to confirm and determine the topographic location of the infarcts, but also because of its utility in their acute treatment. Diffusion-weighted images (DWI) show the infarcted tissue in the acute phase and perfusion-weighted images (PWI) make it possible to quantify the hypoperfused tissue; the difference between the 2 zones will identify the penumbral tissue or mismatch, which is potentially salvageable with recanalization therapy (Figure 3). Cranial MR is recommended for locating and confirming lacunar infarcts, and assessing vertebrobasilar territory stroke. The study should be completed with MR angiography or, in exceptional cases, with intravenous digital subtraction arteriography (IVDSA) when Doppler ultrasound of the supraaortic trunks and/or CT reveals stenosis greater than 50% in internal carotid artery or when intracranial stenosis is suspected.
Figure 3. Ischemic penumbra or mismatch. Ischemic stroke in the middle cerebral artery territory, with occlusion of the artery on magnetic resonance angiography (MRA). Top: diffusion-weighted image (DWI) of the infarct lesion and penumbral tissue detected in a perfusion-weighted image (PWI) corresponding to the ischemic area. The difference between the 2 constitutes the penumbra or mismatch. Bottom: images obtained 24 hours later showing arterial recanalization of the ischemic penumbra with residual lesion in DWI.
TREATMENT
Stroke is a medical emergency that requires immediate intervention. Cerebral infarction is progressively established over several hours, and the size of the infarct can be minimized if we act within that therapeutic window.
In recent years, there has been a substantial change in the therapeutic approach to ischemic stroke. The concept of ischemic stroke as a process that progresses over a period of hours has provided new perspectives, with drugs for recanalization and neuroprotective agents, and with medical treatment that is more effective in preventing greater neuronal damage. The proper evaluation of the cause of the stroke, its pathophysiology and vascular topography will determine the ideal treatment and, consequently, improve the prognosis. The application of these diagnostic and therapeutic measures according to existing protocols within the first 6 hours of the onset of symptoms significantly reduces disability and shortens the hospital stay.11
The discovery of thrombolytic therapy, together with the implementation of stroke units (increased vigilance and monitoring during the first few days), has been the most important advance in the treatment of stroke patients in recent years.
The treatment of stroke is based on 2 concerns: acute treatment and the prevention of recurrences. Acute treatment can be divided into 2 parts: general measures and recanalization therapy.
General Measures: Stroke Units
General care has been shown to be effective in improving the prognosis in stroke patients. Hypoxia, severe hypertension, or hypotension, hyperglycemia, hypothermia, dehydration, and malnutrition are factors that have a negative influence on the functional prognosis in stroke as they provoke greater neuronal damage.12-17 The monitoring of vital signs and the treatment and early detection of these complications, as well as early mobilization, are all measures included in the guidelines and recommendations for stroke care.2
Stroke units consist of multidisciplinary teams, coordinated by neurologists with specialized training in cerebrovascular diseases, providing immediate care, with continuous availability of diagnostic techniques such as cranial CT, transcranial duplex Doppler involving the supraaortic trunks and, frequently, diffusion and perfusion MR imaging, and MR angiography.
Stroke units should be equipped with 4 to 8 beds, according to the needs, and provide acute and nonintensive care. There the patients are monitored continuously for the early detection and correction of factors that may worsen the acute ischemic process, and can receive specific treatment for cerebral infarction. In these units, continuous observation will be maintained by the nursing staff. A standardized protocol for diagnostic evaluation, treatment, and rehabilitation will be followed. Protocols should also be adopted for thrombolytic therapy, prevention of the complications of stroke (cerebral edema, epileptic seizures), prevention of medical complications (control of blood glucose, arterial blood pressure, and body temperature, prevention of deep venous thrombosis, detection of arrhythmias, and respiratory tract infections, nutritional care, prevention of decubitus ulcers), and early rehabilitation.
This multidisciplinary specialized care is the basis for the concept of stroke units. The development of these units has resulted in a significant reduction in mortality and morbidity rates, the hospital stay and costs.18-22 We have found similar results, with an improved prognosis in patients with severe stroke with respect to the conventional hospital setting.23 The metaanalysis of the Stroke Unit Triallists' collaboration24 demonstrated a reduction of 18% in the relative risk in terms of mortality, morbidity and disability, and the benefits were the same for all types of stroke and all age groups.
Recanalization Therapies: Thrombolysis
Intravenous Thrombolysis
The purpose of thrombolytic therapy is to achieve early restoration of arterial flow and preserve the reversibly damaged neuronal tissue in the ischemic penumbra, by means of a relatively safe agent, in order to improve the disease course. Reducing neuronal damage results in less severe functional disability.
The United States Food and Drug Administration (FDA) approved the use of recombinant tissue plasminogen activator (rt-PA) as the first-line treatment for ischemic stroke in June 1998, and in 1999, Canada followed suit. In March 2003, the utilization of rt-PA within the first 3 hours after the onset of stroke was approved by the European Medicines Agency, although it was conditional on the phase IV SITS-MOST study, which has been completed, and the results of which were recently published.25
The reported benefits are based on the results of the NINDS study,26 which demonstrated that the use of rt-PA within the first 3 hours produced an absolute increase of 11% to 13% in the number of patients with an excellent outcome. In comparison with the patients who received placebo, those treated with rt-PA had a 30% higher possibility of being asymptomatic or having a minimal disability 3 months after the stroke. This benefit is not accompanied by an increase in mortality, although it is associated with an incidence of symptomatic cerebral hemorrhage of 6.4%, versus 0.6% in the placebo group.
This finding emphasizes the fact that treatment should be administered by professionals with a complete understanding of its use. The European Medicines Agency recommends its administration by personnel with expertise in the treatment of acute stroke. A number of prospective phase IV studies in which the NINDS treatment criteria27 are utilized and the routine clinical practice in Spanish hospitals (Spanish registry) have observed similarly favorable results28 (Figure 4).
Figure 4. Results of the ECASS II (<3 hours) study, the NINDS study, the Spanish registry, and the SITS-MOST study.
The results of the phase IV SITS-MOST study, carried out at the behest of the European Medicines Agency, have recently been published. The study involved 6483 patients from 285 centers in 14 countries. It demonstrated definitively the efficacy of intravenous thrombolytic therapy with rt-PA administered within the first 3 hours of the onset of stroke; 54.8% of the treated patients were independent of caregivers 3 months later, and the incidence of symptomatic hemorrhages was 7.3%, which is even lower than the overall rate observed in clinical trials (8.6%).25
Two European studies, ECASS I and ECASS II,29 found no benefits when the window was extended to 6 hours, although there is scientific evidence of its efficacy up to 4 and a half hours after the onset of stroke, an approach that is presently being evaluated in the clinical trial, ECASS III (presented at the 2003 International Stroke Conference).
All the studies with streptokinase revealed an unacceptable risk of hemorrhage.30
Intraarterial Thrombolysis
Intraarterial treatment with prourokinase in middle cerebral artery occlusion has been found to be safe and effective when administered within 6 hours of the onset of symptoms (PROACT II study).31 The FDA has not approved the use of prourokinase because it never carried out a confirmatory study, although it is used in routine clinical practice for the intraarterial treatment of basilar artery occlusion.
The most important limitation to intraarterial thromobolytic therapy is the need to perform emergency superselective angiography, which requires an interventional neurologist or neuroradiologist and a special stroke care team. The new approaches include combination therapy, with initial treatment with intravenous rt-PA at the local hospital, followed by rapid transfer to the stroke treatment center for angiography and assessment of the benefits of intraarterial fibrinolysis, if recanalization has not taken place.32
Antiplatelet and Anticoagulation Therapy During the Acute Phase
The administration of aspirin 48 hours after the occurrence of stroke reduces the mortality and recurrence rates, according to 2 large unblinded studies.33,34
There is no scientific evidence of the benefits of early anticoagulation during the acute phase of stroke,35 although it is a routine clinical practice in certain situations, such as recurrent TIA, arterial dissection, basilar thrombosis, and suspected ischemic stroke of cardioembolic origin when there is high risk for reembolization (artificial valves, atrial fibrillation, acute myocardial infarction [AMI] with wall thrombus).
In the case of venous thrombosis, prophylaxis with low molecular weight heparin is always recommended.
New Therapies
Glycoprotein IIb/IIIa Inhibitors
The glycoprotein IIb/IIIa inhibitors block the final common pathway of platelet aggregation and also reduce the release of tissue factor, which activates thrombin formation.36 They have been shown to be effective in the prevention of thrombus formation in acute coronary syndrome and in percutaneous interventions. A phase IIb trial37 pointed out the efficacy of said treatment in patients with ischemic stroke within the first 6 hours of onset. There are studies underway to assess the association of fast-acting antiplatelet agents to prevent reocclusion after treatment with rt-PA (www.strokecenter.org/trials).
Prolongation of the Therapeutic Window With New Thrombolytic Agents: Desmoteplase
Most stroke patients are not candidates for fibrinolytic therapy with rt-PA because the 3-hour time limit has elapsed. The application of treatments with greater specificity for fibrin and a better selection of patients with penumbral tissue by means of MR-DWI/PWI can prolong the therapeutic window without increasing the risk of cerebral hemorrhage and, thus, make treatment available to a greater number of patients. Desmoteplase, a molecule derived from the saliva of the vampire bat (Desmodus rotundus), is a plasminogen activator that has higher affinity for fibrin and, consequently, the risk of hemorrhage at sites distal to the thrombus is lower. A phase II study has been carried out in ischemic stroke patients with mismatch on cranial MR who were treated three to 9 hours after the onset of the event,38 with favorable results in terms of recanalization in 71.4% of the treated patients (P=.0012), versus 19.2% in the placebo group. Likewise, the functional prognosis was favorable at 90 days and the incidence of cerebral hemorrhage was low, 2.2%, with doses of 125 µg/kg. The attempt will be made to confirm these excellent results in the DIAS-II study, the results of which will be published in 2007.
Mechanical Lysis
Mechanical treatments could obviate or complement the need of thrombolytic agents that increase the risk of hemorrhage and, thus, could be highly interesting. Endovascular mechanical thrombectomy has been found to be feasible and safe, and the results are favorable.39 The combination of mechanical thrombectomy with intraarterial thrombolysis could prove to be a promising treatment,40 with favorable results even in the subgroup of patients with internal carotid artery occlusion,41 which is associated with a worse prognosis and a poor response to intravenous fibrinolytic therapy. The results of the Mechanical Embolus Removal in Cerebral Ischemic (MERCI) study have led to the approval of the MERCI Retrieval System, although this therapy has not been approved for the treatment of stroke.
Ultrasonography
Experimental studies, both in vitro and in animal models, have demonstrated the thrombolytic effect of ultrasounds. The application of ultrasounds over the occluded artery in patients with cerebral infarction is a routine practice in the diagnostic management by means of transcranial Doppler. The CLOTBUST study42 has demonstrated that the application of ultrasonography by means of transcranial Doppler, which is systematically employed for the diagnostic study, over the occluded artery, together with the simultaneous administration of rt-PA, improves the rate of recanalization and the patient outcome.
Neuroprotection
The purpose of neuroprotection is to block the biochemical changes, the "ischemic cascade," produced in the penumbral tissue, which lead to cell death.
Over the past 10 years, numerous neuroprotective agents have been studied, with promising results in the experimental setting, but with no convincing results in clinical trials. A metaanalysis involving the administration of citicoline to ischemic stroke patients within 24 hours of the event has shown that the rate of recovery in those who received 2000 mg of citicoline a day was 38% higher than that of the placebo group.43
At the present time, a multicenter, double-blind clinical trial involving oral citicoline is underway to confirm these results.
Hypothermia
Hypothermia is the ideal neuroprotector, as it acts at every step of the ischemic cascade. A number of studies have demonstrated that mild hypothermia reduces the ischemic damage because it lowers the energy requirements and inhibits the production of free radicals and neuroexcitatory amino acids.44 Some of the studies performed in patients have shown that moderate hypothermia during the acute phase of stroke is feasible and can be beneficial.45,46
Preventive Treatment
The strategies for preventing stroke recurrence can be subdivided into 2 large groups: those specific to the stroke subtype and the underlying mechanism, and general approaches to deal with the vascular risk factors.
A number of risk factors increase the possibility of recurrence. Table 3 shows the most common and widely recognized ones, their prevalence, and the relative risk.
The optimal treatment of these factors would markedly reduce the risk of stroke recurrence and that of other vascular complications.
The main strategies for preventing recurrences are as follows:
1. Antiplatelet agents for ischemic stroke not secondary to cardioembolic events (atherthrombotic and lacunar strokes, and strokes of undetermined etiology).
2. Anticoagulation in cardioembolic ischemic strokes.
3. Surgical treatment for symptomatic carotid stenosis.
4. Control of hypertension.
5. Control of hyperlipidemia.
6. Control of diabetes mellitus.
7. Changes in habits (smoking, alcohol consumption, physical exercise, and control of obesity).
8. Treatment of systemic hematological diseases, which can cause stroke, although they are not among the more common factors.
Antiplatelet Agents
Antiplatelet agents are the drugs most widely used for secondary prevention of ischemic stroke, except that of cardioembolic origin and certain uncommon subtypes, such as arterial dissection and other systemic diseases.
Acetylsalicylic acid (ASA) has been found to be effective in the prevention of stroke, TIA, AMI, and vascular death, with a reduction in the relative risk of 25% as compared to placebo.48 In patients with a history of stroke, a reduction of 23% is reported.
No significant differences have been observed among the doses employed in terms of efficacy, but higher doses are associated with a greater risk of hemorrhagic complications.49-51 At the present time, it is the treatment of choice at doses of 100-300 mg/day.2,52,53
Clopidogrel
Clopidogrel is indicated in patients at high vascular risk or with intolerance to ASA. There is a reduction in the relative risk of stroke, AMI, and vascular death of 8.7% as compared to ASA, and a lower incidence of gastrointestinal hemorrhage, and the absolute benefit is much greater than that obtained with aspirin if we analyze the subgroup of patients with a previous history of vascular disease (stroke or AMI).54,55
There are other antiplatelet agents, such as ticlopidine, triflusal, and dipyridamol, but they are much less widely used and the indications are more limited.
Combination of Clopidogrel and Acetylsalicylic Acid
The MATCH and CHARISMA studies have demonstrated no benefits in the prevention of the recurrence of cerebrovascular events, but did find an excessive risk of hemorrhage. Currently, this treatment is not recommended in stroke patients.56
Anticoagulants
Anticoagulation with oral anticoagulants is the treatment of choice for the prevention of cardioembolic stroke. The European Atrial Fibrillation Trial and the Stroke Prevention in Atrial Fibrillation Trial have demonstrated that warfarin with an international normalized ratio (INR) of 2 to 3 is superior to ASA in the prevention of recurrent stroke in patients with atrial fibrillation.57,58
The treatment of stroke due to right-to-left shunt secondary to PFO warrants separate consideration. We have found no specific studies designed to evaluate the different therapeutic possibilities. In secondary prevention of stroke associated with PFO, with or without atrial septal aneurysm, antiplatelet therapy, anticoagulation, and endovascular closure have been used. At the present time, the risk of recurrence in these patients is unknown. Preliminary data from the Spanish cooperative study, CODICIA, show a low rate of recurrence and a good prognosis with respect to stroke (European Stroke Conference 2006). The results from the PICSS study, a substudy of the WARSS study,59 do not show anticoagulation to be superior to antiplatelet therapy either, and there are no other available studies comparing the efficacy of antiplatelet therapy with anticoagulation or percutaneous of PFO.60 Several reports indicate that endovascular closure would be the best therapeutic option, although implantation of these systems also has its risks. These studies are retrospective and there are no long-term studies or clinical trials that demonstrate it to be superior to medical treatment.61 Current recommendations accept the use of anticoagulants or percutaneous closure in cases of recurrence in which antiplatelet therapy has failed.2,52,53 Given the secondary effects of anticoagulation therapy, its cumulative effect and the lack of scientific proof of its superiority with respect to antiplatelet therapy, its use should be avoided. In selected young patients with recurrent stroke and the coexistence of PFO and atrial septal aneurysm, percutaneous closure should be considered.
Hypertension
Hypertension (HT) is the most important risk factor for stroke. The arterial pressure should not be lowered suddenly during the first 48 hours after stroke, unless the patient has heart or renal failure, since a sharp decrease during the initial hours can increase the severity of the stroke.
The most important factor associated with the decrease in the risk of stroke recurrence is the control of the arterial pressure. The dose-response relationship between HT and the risk of stroke is continuous.62,63 The desired blood pressure level is 130/80 mm Hg, although lower levels arebeneficial in terms of prevention.
Proper treatment of arterial pressure appears to be much more determinant than the choice of the drug, although recent studies appear to demonstrate the efficacy of angiotensin converting enzyme (ACE) inhibitors, with or without diuretics, as well as angiotensin II receptor antagonists. These drugs have beneficial effects beyond that related to blood pressure control, possibly because of their endothelial effects. The HOPE study, with ramipril (10 mg/day),64 reported a more marked reduction in cerebrovascular events, both in primary and secondary prevention, in patients at high vascular risk. The PROGRESS trial shows favorable results, both in hypertensive and normotensive patients in secondary prevention, with the combination of perindopril (4 mg) and indapamide, as compared to perindopril alone plus placebo.65 Angiotensin II receptor antagonists also appear to play an important role in prevention beyond the control of blood pressure, as shown in the LIFE study, which compared losartan with atenolol in hypertensive patients with left ventricular hypertrophy, with favorable results in primary prevention of cerebrovascular events,66 and in the MOSES study, which reported favorable results with eprosartan (600 mg) versus nitrendipine (10 mg) in hypertensive patients with a history of cerebrovascular events.67
Hypercholesterolemia
Although hypercholesterolemia has been shown to be a risk factor for ischemic heart disease, its association with ischemic shock has been controversial and confusing. However, recent studies indicate an association between high cholesterol levels and ischemic stroke and, thus, suggest the need to follow the recommendations of the National Cholesterol Education Program III (NCEPIII),68 in which, the level of low-density lipoprotein cholesterol at which treatment, consisting of statins, should be introduced is established according to the degree of vascular risk (on the basis of the presence of the following factors: smoking habit, HT, high-density lipoprotein cholesterol level less than 40 mg/dL, family history of early-onset ischemic heart disease and age of 45 years in men and 55 years in women). Scientific evidence from clinical trials with statins in patients with ichemic heart disease and the metaanalysis of these trials show a significant decrease in the risk of stroke.69,70 These results have led the FDA to approve the utilization of pravastatin and simvastatin in the prevention of stroke in patients with concomitant ischemic heart disease.71 Overall, the use of these drugs does not increase the risk of cerebral hemorrhage.
According to recent recommendations, the objective in patients with coronary artery disease or equivalent will be to maintain low-density lipoprotein cholesterol levels under 100 mg/dL; the presence of symptomatic atheromatous carotid stenosis is one of these equivalent diseases.
The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) study72 was designed to evaluate the efficacy of atorvastatin in the secondary prevention of stroke recurrence in patients with prior stroke without ischemic heart disease, and it is the only clinical trial dealing specifically with secondary stroke prevention. The recently published results of the SPARCL study show a reduction in the risk of stroke of 16% (odds ratio [OR], 0.84; 95% confidence interval [CI], 0.71-0.99; P=.03), in the risk of ischemic stroke of 23% (OR, 0.78; 95% CI, 0.66-0.94; P=.007), and in coronary artery disease of 43% (OR, 0.57; 95% CI, 0.35-0.95; P=.003) versus placebo, with atorvastatin at a dose of 80 mg/day.
Treatment of Atherothrombotic Carotid Stenosis
The final results of the ECST73 and NASCET74 studies have demonstrated the efficacy of carotid endarterectomy, under certain conditions, in the prevention of recurrence in patients with symptomatic carotid stenosis greater than 70% who have experienced a TIA or minor stroke (grade A recommendation, based on type I evidence).
According to the final results of the NASCET study, surgical treatment is indicated in symptomatic carotid stenosis when it is equal to or greater than 70%, and probably for stenosis of 60% or greater if we consider the results of the European ECST study. Figure 5 shows the equivalence between the two studies.
Figure 5. Quantification of the degree of carotid stenosis. Correlation between the NASCET and ECST methods. CCA indicates common carotid artery.
Angioplasty or Endarterectomy in Carotid Stenosis: Present Status
The NASCET and ECST studies, on which the indication for carotid endarterectomy is based, have been criticized and questioned with respect to the possibility of reproducing their results in routine clinical practice. As the centers and surgeons were selected with great care, the generalization of the results to other centers and surgeons that probably have less experience and lower quality standards is questioned. Percutaneous transluminal angioplasty is emerging as an alternative to carotid endarterectomy, considering that the surgical morbidity and mortality (5.8%) should not be disregarded and that the risk/benefit margin with respect to medical treatment is very narrow. The CAVATAS (Carotid and Vertebral Artery Transluminal Angioplasty) study75 demonstrated that the morbidity and mortality associated with carotid endarterectomy and percutaneous transluminal angioplasty are similar, and that the benefits obtained at 3 years do not differ significantly from those reported in the NASCET and ECST studies. Thus, carotid angioplasty has not been found to offer greater benefits, in terms of outcome, than endarterectomy and, although it should be reserved for cases in which endarterectomy is contraindicated, it would be an excellent option in teams with experience and low morbidity and mortality rates. Studies underway show increasingly lower rates of complications and, thus, it is very possible that, in the near future, its range of applications will become broader. This decrease in morbidity and mortality is due, on one hand, to the learning curve, but especially to the introduction of modifications in the technique, such as the placement of a metal stent. The stent has a mesh configuration that prevents intimal dissection, a circumstance that reduces the incidence of restenosis and distal emboli following inflation of the angioplasty balloon. Another element that has played a role in the decrease in distal embolic complications is the placement of a protective "umbrella" which, positioned distally during dilatation, acts as a filter for fragments that may become detached from the plaque.
Table 4 shows the current indications for carotid angioplasty. Today, angioplasty should be considered an experimental technique that should be employed in the context of clinical studies or trials. Nevertheless, we should consider its use in patients who do not qualify for surgery for anatomical reasons or due to the coexistence of multiple risk factors that could increase morbidity and mortality in endarterectomy (cumulative vascular risk factors, contralateral stenosis, or occlusion, distal stenoses), or because they can not receive general anesthesia.
On the other hand, angioplasty may also be contraindicated due to anatomical factors (heavily calcified stenoses or highly tortuous vessels) or to elements that increase the risk of stroke, as is the case of preocclusive stenoses or pseudoocclusions, or in critical stenoses with unorganized thrombi, in which the catheter that has to pass through the stenosis might detach them and provoke distal emboli.
Surgical Indications in Asymptomatic Stenoses: Primary Prevention
One of the most controversial issues is the performance of carotid endarterectomy in patients with asymptomatic carotid stenosis. The ACAS study77 and, more recently, the results of the ACST study,78 show that endarterectomy is superior to medical treatment in patients with asymptomatic carotid stenosis greater than 70% (NASCET criteria). However, despite the statistical significance observed, the absolute difference with respect to primary events at 5 years was 5.8%, requiring the performance of nearly 85 endarterectomies to prevent 1 stroke over 2 years, provided that the morbidity and mortality rates of the team are less than 3%. These figures, with the morbidity and mortality called for, in a trial carried out by surgical teams selected on the basis of very strict criteria in which extensive experience and high efficacy were required, makes the generalization of these results in routine practice highly difficult. For this reason, it is necessary to be very strict with respect to the indications for endarterectomy in asymptomatic carotid stenosis. This aspect is highly evident if we consider that, in symptomatic patients, the number needed to treat (NNT) is 7 to 8 endarterectomies in order to prevent 1 stroke (Table 5).
One aspect that is currently under development is the application of techniques that enable the identification of the patients at highest risk. This would, in the near future, make it possible to identify and classify patients with carotid stenosis into 2 groups: low-risk (medical treatment) and high-risk (endarterectomy or angioplasty).
HEMORRHAGIC STROKE
Hemorrhagic stroke constitutes 15% to 20% of all strokes, with a 30-day mortality of 35% to 52% in which half of the deaths occur within the first 2 days.80,81 Early diagnosis, proper treatment and prevention will determine the course and prognosis.
Hemorrhagic stroke includes intracerebral hemorrhage (ICH), a collection of blood within the brain parenchyma caused by spontaneous, nontraumatic vascular rupture, and subarachnoid hemorrhage (SAH), defined as bleeding within the subarachnoid space.
Hemorrhagic stroke is that which occurs most frequently in patients under 40 years of age, and it is 2 to 3 times more common among blacks and Asians.82
Hypertension is the most common cause of nontraumatic ICH (60%).83 Intracerebral hemorrhage can also be secondary to coagulopathy, to thrombolytic therapy or to anticoagulants in 6% to 10% of the cases. Antiplatelet therapy has been associated with a greater tendency, although not statistically significant, toward ICH. Cerebral amyloid angiopathy is the cause of 5% to 10% of the cases of ICH, and is more common in older patients with cognitive deterioration. The diagnosis is based on neuropathological findings and is suspected in the presence of recurrent hemorrhages in older patients with cognitive deterioration. Arteriovenous malformations and aneurysms can also cause ICH. In addition, ICH can be provoked by highly vascularized brain tumors, such as melanoma metastases, hypernephroma, and choriocarcinoma.
In 90% of the cases, SAH is secondary to rupture of a cerebral aneurysm.
Other less frequent causes include vasculitis, venous infarction secondary to cerebral venous sinus thrombosis, central nervous system infections, and cocaine and amphetamine use.
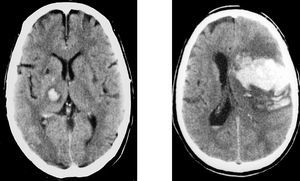
Fifty percent of ICH are located deep in the cerebral hemispheres, in the basal ganglia (putamen, internal capsule, caudate nucleus, and thalamus). Hypertension is implicated in most of them.
Forty percent of ICH are lobar, the majority in parietal and occipital lobes. In lobar ICH, aside from HT, other etiologies, such as vascular malformation in young patients, metastases and cerebral amyloid angiopathy in older patients, should be considered (Figure 6).
Figure 6. Intracerebral hemorrhage. Left: deep intracerebral hypertensive hemorrhage (thalamocapsular). Right: lobar intracerebral hemorrhage.
The remaining 10% are infratentorial, cerebellar, or pontine.
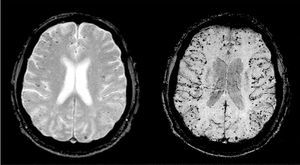
Neuroimaging is essential for the diagnosis of hemorrhagic stroke. It is suspected in patients with acute focal neurological symptoms, and the bleeding is detected by cranial CT or MR. Specific gradient-echo MR sequences that detect the paramagnetic effects of deoxyhemoglobin and methemoglobin enable the discovery of chronic parenchymal bleeding (microbleeds) (Figure 7).
Figure 7. Microbleeds. Gradient-echo magnetic resonance sequences showing cortical microbleeds compatible with cerebral amyloid angiopathy.
Cerebral arteriography is indicated in the case of SAH and when vascular malformations are suspected.
Important advances have been made in recent years, with a better understanding of the pathophysiology of and the factors implicated in ICH and the neurological deterioration,84-87 medical treatments to limit the growth of the hematoma88 and new surgical techniques that offer promising prospects for the future of this disease.89
The monitoring of vital signs (as in ischemic stroke); stabilization of respiratory function and the response to hypoxia; control of arterial pressure, blood glucose and body temperature; prevention and treatment of complications; secondary prevention and early rehabilitation are essential in order to prevent greater neurological damage. Neurological follow-up in a specialized unit is recommended during the acute phase.89
The increase in ICH can contribute to early neurological deterioration and, thus, any coagulation disorder should be rapidly corrected. In patients with a normal coagulation profile, early hemostatic therapy will help to minimize the increase in hematoma volume and improve the prognosis.90 Recombinant activated factor VII, administered within 4 hours of the clinical onset is the only treatment that has been proved to be safe and effective. A randomized, prospective, placebo-controlled study involving 400 patients demonstrated limited hematoma growth, decreased mortality, and a better functional prognosis, when compared to placebo.88
Surgical treatment has yet to be defined and is only accepted in certain cases.8,89 In cerebellar hemorrhage greater than 3 cm, with trunk compression and hydrocephalus, early surgical drainage is recommended. Placement of a ventricular drain should be carried out in cases of ICH with secondary hydrocephalus. In SAH, the early placement of coils to repair the ruptured aneurysm is recommended.
In spontaneous ICH, the results of surgery in deep basal ganglia hemorrhages are not conclusive. In lobar hemorrhage, depending on the location, the status of the patient and the size of the hemorrhage, it may be an option to be considered, although the scientific evidence in this respect is insufficient. In the STICH trial,91 in which early surgical intervention was compared with initial conservative treatment, considerable differences were not observed.
Young patients with lobar hemorrhage who exhibit neurological deterioration due to growth of the hemorrhage can be considered candidates for surgical drainage.8,89
The surgical techniques are advancing and new procedures, such as small craniectomies guided by neuronavigation systems, microsurgical techniques, or endoscopic evacuation, may produce promising results.89
Section sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. J. Serena Leal.
Servicio de Neurología. Hospital Universitari Dr. Josep Trueta.
Avda. França, s/n. 17007 Girona. España.
E-mail: nrl.jserena@htrueta.scs.es