Keywords
INTRODUCTION
This article is the customary annual update analysis describing results of heart transplantation (HT) activity conducted in Spain between the first such procedure, performed in May 1984, and December 31, 2009.1-20
This Registry includes data on all HTs performed by teams at all centers in Spain (Annex 1). It is, therefore, an accurate account of the status of heart transplantation in the country. The report's reliability is founded on the nationwide use of a single database constructed on mutually agreed principles, which standardizes variables and the possible responses.
METHODS
Patients and Centers
Nineteen HT centers (Table 1) have supplied the Registry with data, although only 18 are currently active.
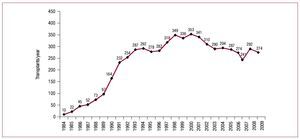
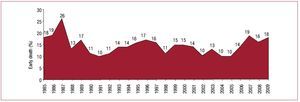
In the 25 years that HT procedures have been performed in Spain, the total number of operations has reached 6048. Figure 1 presents the distribution of the number of HTs per year. Of these, 94% were isolated orthotopic transplants. Table 2 shows the distribution of transplants by procedure type.
Figure 1. Number of heart transplants per year.
Design
The database includes 175 clinical variables with data on recipients, donors, surgery, immunosuppression and follow-up. Each year, the centers send data to the Registry Director, who organizes the statistical methodology with the company contracted to perform the analysis. The Director is also responsible for organizing the audit of centers to verify data which is conducted by an independent external company that randomizes the centers and HTs, extracts a representative sample, and confirms the reliability of the data submitted.
In 2008, the Registry was presented to, and received the approval of, the Committee for Biomedical Research Ethics of the Hospital Universitario La Fe, Valencia. We are in the process of submitting the Registry to the Spanish Ministry of Health and Consumer Affairs to guarantee fulfillment of Spanish Data Protection Law 15/1999.
Statistical Analysis
Variables are presented as mean (SD). Data on survival are analyzed in Kaplan-Meier curves and compared using the log-rank test. A P value of <.05 was considered significant.
RESULTS
Heart Transplant Recipient Profile
In Spain, the profile of the average HT recipient is that of a 53-year-old man diagnosed with ischemic heart disease or idiopathic dilated cardiomyopathy and with blood group A or O. Table 3 shows the clinical profile of isolated HT recipients by age-group; retransplantation recipients appear in a separate column.
Waiting List Mortality and Days to Transplant
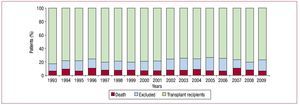
In 2009, waiting list mortality was 7%. The percentage of patients excluded from transplantation after inclusion on the waiting list was 16%. Figure 2 shows the annual percentage of waiting list patients who received an HT, were removed from the list without receiving one, or died before receiving one.
Figure 2. Patient outcomes following inclusion on the heart transplantation waiting list.
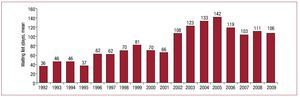
Mean waiting list time for recipients prior to undergoing HT in 2009 was 106 days. Figure 3 shows how this has evolved over the last 18 years.
Figure 3. Year-by-year evolution of mean dayson waiting list for heart transplantation for.
Cause of Death and Mean Donor Age
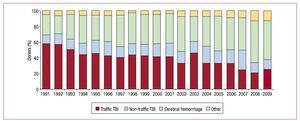
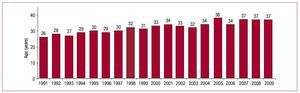
Most HT donors die of cerebral hemorrhage. Mean donor age in 2009 was 37 years (Figures 4 and 5).
Figure 4. Year-by-year evolution of causes of heart transplant donor deaths. TBI, traumatic brain injury.
Figure 5. Year-by-year evolution of mean age of heart transplant donors.
Urgent Transplantation
The rate of indication for urgent transplantation in 2009 was 38%. Figure 6 shows the evolution of indication for urgent HT over the years.
Figure 6. Year-by-year evolution of urgent heart transplantations.
Ventricular Assist Devices
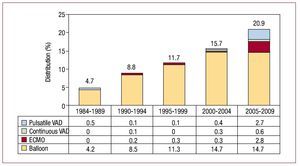
In recent years, the percentage of HT recipients receiving ventricular assist devices has increased, reaching 21% in the last 5 years. Distribution by periods and implanted device type are shown in Figure 7.
Figure 7. Distribution of ventricular assist device types deployed prior to transplantation, by periods. ECMO, extracorporeal membrane oxygenation device; VAD, ventricular assist devices.
Immunosuppression
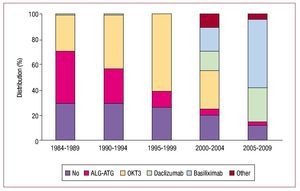
In Spain, most HT recipients receive induced immunosuppression treatment. The drugs used and the distribution by periods appear in Figure 8.
Figure 8. Induced immunosuppression. Drugs administered.
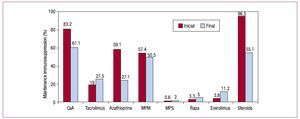
De novo maintenance immunosuppression treatment administered and changes made during HT recipients' clinical course are shown in Figure 9.
Figure 9. Maintenance immunosuppression. Variations in clinical course by drug type. Immunosuppression at transplantation and at end of follow-up. CsA, cyclosporine A; MPM, mycophenolate mofetil; MPS, mycophenolate sodium; Rapa, rapamycin.
Survival
Early mortality (death at ≤30 days post-transplantation) was 18% in 2009 (Figure 10). In 2005, it was 10% and since then it has increased by a mean 7%.
Figure 10. Year-by-year percentage evolution of early deaths (at ≤30 days).
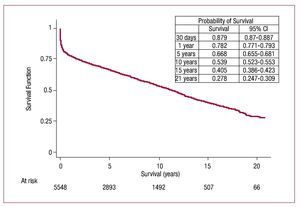
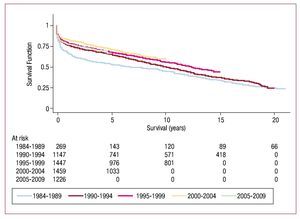
When survival rate data for 2009 were added to those of previous years, we obtained 88% 1-month actuarial survival and 1-, 5-, 10-, and 15-year rates of 78%, 67%, 54% and 41%, respectively (Figure 11). Survival by periods showed better results in the latter stages with survival rates at 1 and 5 years of 85% and 73%, respectively (Figure 12).
Figure 11. Actuarial survival curve (Kaplan-Meier) for the entire series. CI, confidence interval.
Figure 12. Survival curves by time period.
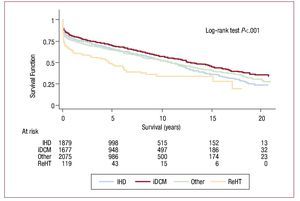
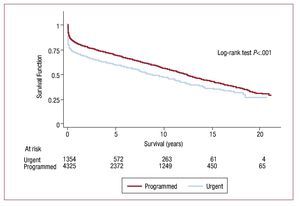
Survival curves differed according to the etiology indicating HT (Figure 13). Degree of urgency influenced probability of survival (Figure 14).
Figure 13. Survival curves according to etiology indicating transplantation. iDCM, idiopathic dilated cardiomyopathy; IHD, ischemic heart disease; ReHT, retransplantation.
Figure 14. Survival curves by degree of urgency.
Causes of Death
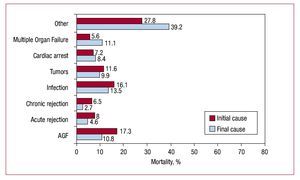
The most frequent cause of death was early graft failure (17%), followed by infection (16%), combined graft vascular disease and sudden death (14%), tumors (12%) and acute rejection (8%) (Figure 15).
Figure 15. Causes of overall mortality, initial cause leading to death, and final cause of death. AGF, acute graft failure.
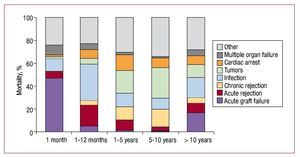
When causes of death are distributed by periods, differences can be seen at ≤30 days (acute graft failure), 1-12 months (infection), and >1 year (tumors, the combination of sudden death with chronic rejection and infection). Figure 16 shows the distribution of causes of death by periods.
Figure 16. Causes of death by post-transplantation period.
DISCUSSION
With 25 years' experience of HT in Spain, and over 6000 transplants performed, we would say that this procedure can be offered to the population at large in the certainty that levels of knowledge, control, and survival are similar to or better than those of other countries in western Europe and around the world. Analysis of the Registry of the International Society for Heart and Lung Transplantation annual report demonstrates this clearly.21-24
An important advantage of the Spanish Heart Transplantation Registry is that it is compiled from a standardized database to which all Spanish Transplant Teams submit data. They update annually and send figures to the Registry Director for collation and submission to an independent statistics consultancy for analysis. We believe this method greatly enhances the reliability of our results and avoids errors of the kind so often found in nonstandardized databases. In 2007, we increased the number of variables analyzed for each patient to 175. In 2008, the Registry received the approval of the Biomedical Research Ethics Committee of the Hospital Universitario La Fe, Valencia.
In the near future, the Registry will be submitted to the Spanish Ministry of Health and Consumer Affairs to give it legal coverage and ensure adequate protection of patient healthcare data. Furthermore, to attain improved quality and greater data reliability, we intend to continue with the audit of centers by independent external companies that guarantee maximum data validity.
Currently, 18 HT centers are active (the most recent incorporation was Hospital Vall d'Hebron, Barcelona, in 2006). The fact that, in Spain, centers are authorized to conduct HTs without adequate needs analysis is of great concern to the Transplant Teams because as the number of optimal donors has shown a clear downward trend, the HT/center ratio is falling, too. The fact that fewer HT procedures are being performed leads to under use of resources in hospitals equipped to conduct a great number of transplants, and to a longer learning process needed to achieve adequate results. The only tangible benefit for patients is the convenience of being able to undergo transplantation without having to travel far from home.
In 2009, the number of HTs performed declined (274 in 2009 vs 292 in 2008). This is not particularly worrying as it fits the pattern of recent years. What is of greater concern is the progressive fall in the number of donors over the last 10 years. There is no single explanation for this reduction but it seems clear that the incidence of death from traumatic brain injury has decreased, whereas care of patients with multiple trauma in specialized units has improved.
Waiting list time for patients to obtain a compatible organ was similar to that of the last 3 years (2009, 106 days; 2008, 111; 2007, 103). In 2009, death while on the waiting list was 7%. However, we should add those patients removed from the list due to severe decompensations and not included again because they die after being removed from the list. According to Spain's national transplant organization, these accounted for some 8% of deaths in 2009.25 Consequently, in 2009 mortality among patients with advanced heart failure and waiting for a heart was 15%.
The clinical profile of patients has not changed in recent years. We analyzed HT recipients in 3 groups (pediatric, adult and retransplantation) as each of these is indicated for transplant for very different clinical causes: pediatric patients are operated for congenital heart disease or idiopathic dilated cardiomyopathy; they have higher pulmonary resistances and present no cardiovascular risk factors. In contrast, patients undergoing retransplantation are usually indicated for graft vascular disease; they present greater organ deterioration and more risk factors. This may be a more accurate explanation for the bad prognosis of these patients than the fact that they undergo a second transplant.
Urgent HT is somewhat controversial, as these operations have specific characteristics (recipients in worse clinical condition, less-than-ideal donors, longer periods of ischemia) that entail a worse prognosis than programmed transplants. In 2009, the percentage of urgent HTs rose by 6% (38% in 2009 vs 32% in 2008). The percentage of patients undergoing urgent HT varies from one geographical area to another and alters substantially from one year to the next. Why indication for urgent HT fluctuates like this is unclear and differences in geographical distribution are also difficult to explain, although low donor numbers and improved care of critical patients (ventricular assist device implants) increase opportunities for urgent HT. Indication for urgent HT has been questioned, given that it offers clearly poorer results. However, the Transplant Teams consider that this option should continue to exist although in a controlled form. To ensure optimal chances of survival for critical patients undergoing transplantation we should bear in mind, as European guidelines on heart failure recommend, that it is better to stabilize heart failure than indicate for urgent HT, and that transplantation should not be considered a treatment for unstable acute heart failure26 (among other things, because of the time taken in locating a donor even in cases as urgent as these).
At HT, the percentage of patients with some type of ventricular assist implant has gradually increased, especially in the last 5 years. The intraaortic counterpulsation balloon is the most frequently used device. The use of extracorporeal membrane oxygenation and pulsatile devices has increased substantially, too. In the last 5 years, approximately half the urgent HT recipients had previously had some sort of ventricular assist implant. These devices are crucial to the care and stabilization of patients with acute heart failure prior to transplantation, so it is advisable that Transplant Teams should have access to them for more critical patients.
Induction immunosuppression is used in most HT procedures. Since transplantation was first performed, the most frequent treatment has been with OKT3 antilymphocyte antibodies (35% of the entire series) although currently interleukin 2 antagonists are more common (85% of HTs performed in the last 5 years). The most frequently used maintenance immunosuppression treatment is known as the triple combination: cyclosporine versus tacrolimus, azathioprine versus mycophenolate mofetil, and steroids. However, depending on patient clinical course, the introduction of other immunosuppressors such as rapamycin, everolimus, mycophenolate acid and, more recently, extended-release tacrolimus, is common. Of these, everolimus use has increased the most.
Incidence of early mortality rose in 2009 (18% in 2009 vs 16% in 2008). This tendency has increased over the last 4 years, which may be related to the greater number of urgent cases and increased use of ventricular assist devices as patients reach HT in a more critical condition. The early period is probably the most important in improving survival, as the survival curve stabilizes after the first months post-transplantation.
Over the years, overall survival has shown a clear trend towards progressive improvement. However, logically, the number of patients included in the Registry each year constitutes a smaller percentage of the total. Thus, the probability of substantial changes in any one year is very remote and analysis of survival over longer periods is more illuminating. In recent years, survival has improved considerably by comparison with earlier periods.
Indication for HT is clearly linked to survival and patients diagnosed with idiopathic dilated cardiomyopathy have a higher survival rate than other HT recipients because they are younger and present fewer cardiovascular risk factors.
The most frequent cause of death is acute graft failure (17%), followed by infection (16%), the combination of graft vascular disease and sudden death (14%), tumors (12%) and acute rejection (8%). However, cause of death is usually related to time post-transplantation. Thus, at ≤30 days, the most frequent cause is graft failure; at 1-12 months, infection and rejection; and later, the combination of sudden death with chronic rejection, infection and tumors. It is important to note that, particularly in recent years, infection seems to be gaining ground as a cause of death, whereas death from acute rejection is less frequent. This imbalance could be due to the over-administration of immunosuppressor drugs that prevent rejection but favor infection.
CONCLUSIONS
In general, early and late survival rate figures are similar to those published in international registry reports and have improved year by year, especially in the last 5 years.
We should continue to try to reduce the high incidence of early graft failure, as this would have a particularly good effect on the probability of immediate post-operative and overall survival.
Given that infection is a greater cause of morbidity and mortality than rejection, we should pay it more attention and situate it among the principle objectives of general studies and clinical drug trials.
ABBREVIATIONS
AGF: acute graft failure
CH: cerebral hemorrhage
HT: heart transplantation
Statistical analysis was conducted by ODDS S.L. with unconditional financial support from Novartis Trasplante.
Correspondence: Dr. L. Almenar Bonet.
Avda. Primado Reig, 189-37. 46020 Valencia. Spain
E-mail: lualmenar@gmail.com