A decade has passed since the first Spanish percutaneous pulmonary Melody valve implant (PPVI) in March 2007. Our objective was to analyze its results in terms of valvular function and possible mid-term follow-up complications.
MethodsSpanish retrospective descriptive multicenter analysis of Melody PPVI in patients < 18 years from the first implant in March 2007 until January 1, 2016.
ResultsNine centers were recruited with a total of 81 PPVI in 77 pediatric patients, whose median age and weight were 13.3 years (interquartile range [IQR], 9.9-15.4) and 46kg (IQR, 27-63). The predominant cardiac malformation was tetralogy of Fallot (n = 27). Most of the valves were implanted on conduits, especially bovine xenografts (n = 31). The incidence of intraprocedure and acute complications was 6% and 8%, respectively (there were no periprocedural deaths). The median follow-up time was 2.4 years (IQR, 1.1-4.9). Infective endocarditis (IE) was diagnosed in 4 patients (5.6%), of which 3 required surgical valve explant. During follow-up, the EI-related mortality rate was 1.3%. At 5 years of follow-up, 80% ± 6.9% and 83% ± 6.1% of the patients were free from reintervention and pulmonary valve replacement.
ConclusionsMelody PPVI was safe and effective in pediatric patients with good short- and mid-term follow-up hemodynamic results. The incidence of IE during follow-up was relatively low but was still the main complication.
Keywords
Advances in cardiac surgery, percutaneous catheterization, intensive care, and noninvasive imaging techniques have improved life expectancy and reduced the number of congenital heart disease-related reinterventions.1 Accordingly, and due to the harmful effects of chronic dysfunction of the right ventricular (RV) pulmonary valve, the recent focus of clinical practice has been to shorten intervention times.210.1016/j.recesp.2017.07.00510.1016/j.recesp.2017.07.00510.1016/j.recesp.2017.07.005
Percutaneous pulmonary valve implantation (PPVI) was described for the first time in 2000.3 It is now fairly widely accepted and more than 10 000 such implants have thus far been performed worldwide. The characteristics of the valve and implant system are described in Figure 1. Various devices are currently in use but only the Melody valve (Medtronic; Minnesota, United States) had been approved by the relevant regulatory bodies (CE 9/2006 and FDA 01/2010) at the study start date.
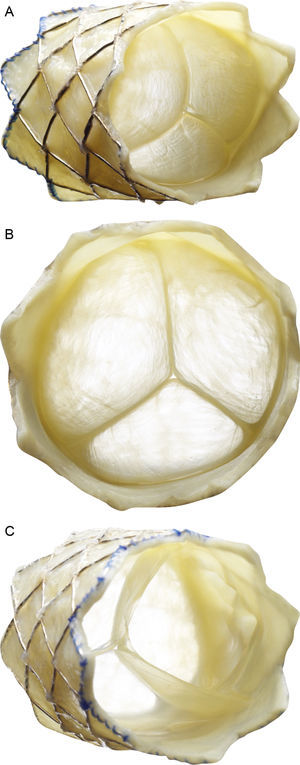
Description of the Melody percutaneous pulmonary valve (Medtronic Inc.; Minneapolis, Minnesota, United States). The valve consists of a 34-mm bare-metal platinum-iridium stent (CP stent, NuMED Inc.; Hopkinton, New York, United States) with a hand-sewn valved segment of bovine jugular vein. A: frontolateral view. B: frontal view. C: view from the distal end. The device is crimped over a balloon-in-balloon catheter and released via a long modified 22-Fr sheath (Ensemble Delivery System, Medtronic Inc.), available in external balloon diameters of 18, 20, and 22mm. The balloon-in-balloon technique permits 2-step valve release and its repositioning once the lower-size balloon is inflated.
The main aim of our study was to present the Spanish experience with Melody PPVIs in pediatric patients and, as a secondary objective, to evaluate its changes over time in terms of valve functioning and any short-to-mid-term complications.
METHODSThis study comprised a descriptive, retrospective, and multicenter analysis of the Melody PPVI in individuals younger than 18 years from March 2007 (date of the first implant in Spain) to January 1, 2016. The Cardiac Catheterization Working Group of the Spanish Society of Pediatric Cardiology and Congenital Heart Disease developed a questionnaire that was subsequently completed by all centers with pediatric PPVI experience. The study was performed in accordance with the Spanish Law on Data Protection 15/1999. Patient follow-up was completed on May 15, 2016.
Major complications were defined as those with clinical impact, those that endangered the patient's life, and those that required surgical or percutaneous treatment (eg, coronary compression, cardiac structure perforation, embolization of prosthetic material). All other complications were considered minor.4 Intraprocedural complications were events occurring from patient catheterization to transfer to intensive care; acute complications were those that occurred up to 72hours afterward.
Statistical AnalysisStatistics/Data Analysis 13.1 (Stata, StataCorp LP, United States) was used for the statistical analysis. Normally distributed quantitative variables are expressed as mean±standard deviation; those with a nonnormal distribution are expressed as median [interquartile range]. Nominal variables are expressed as numbers and percentages; the Student t test was used to compare differences between pre- and postimplantation means. The chi-square test, Wilcoxon test, and Mann-Whitney U test were used as required to compare categorical variables. All tests were 2-sided and a P value less than .05 was considered statistically significant. Analysis of event-free survival was performed using Kaplan-Meier curves. Factors associated with an event (reintervention/endocarditis) during follow-up were included in univariable Cox regression analysis and were considered significant at P ≤ .05.
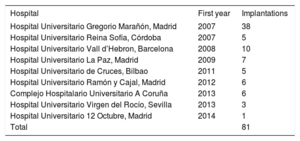
RESULTSSince the first implant in Spain until January 1, 2016, a total of 173 Melody valves were implanted in 16 hospitals; of these, 81 were used for PPVI in 77 pediatric patients (Table 1). One patient underwent a double implantation because he had a double right ventricular outflow tract (RVOT) (conduit+native RVOT) and 3 patients required a second Melody valve (2 via the valve-in-valve technique and the other after explantation of the initial valve and surgical reconstruction of the RVOT).
List of National Hospitals With Experience in Melody Percutaneous Pulmonary Valve Implantation, in Order of Year of First Implantation
| Hospital | First year | Implantations |
|---|---|---|
| Hospital Universitario Gregorio Marañón, Madrid | 2007 | 38 |
| Hospital Universitario Reina Sofía, Córdoba | 2007 | 5 |
| Hospital Universitario Vall d’Hebron, Barcelona | 2008 | 10 |
| Hospital Universitario La Paz, Madrid | 2009 | 7 |
| Hospital Universitario de Cruces, Bilbao | 2011 | 5 |
| Hospital Universitario Ramón y Cajal, Madrid | 2012 | 6 |
| Complejo Hospitalario Universitario A Coruña | 2013 | 6 |
| Hospital Universitario Virgen del Rocío, Sevilla | 2013 | 3 |
| Hospital Universitario 12 Octubre, Madrid | 2014 | 1 |
| Total | 81 |
To homogenize the data, the results of the 78 primary implantations (77 patients) were analyzed. Clinical descriptions of the patients and the procedure are provided in Table 2 and Table 3, respectively. The modern technique of prestenting 2 was used in all procedures, except for implantations performed for a dysfunctional surgical valve prosthesis (6 patients) or for the initial experiences (7 patients).
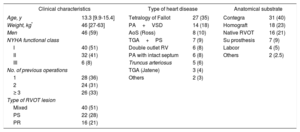
Characteristics of the 78 Percutaneous Pulmonary Valve Implantations in 77 Patients Younger Than 18 Years
| Clinical characteristics | Type of heart disease | Anatomical substrate | |||
|---|---|---|---|---|---|
| Age, y | 13.3 [9.9-15.4] | Tetralogy of Fallot | 27 (35) | Contegra | 31 (40) |
| Weight, kg* | 46 [27-63] | PA+VSD | 14 (18) | Homograft | 18 (23) |
| Men | 46 (59) | AoS (Ross) | 8 (10) | Native RVOT | 16 (21) |
| NYHA functional class | TGA+PS | 7 (9) | Su prosthesis | 7 (9) | |
| I | 40 (51) | Double outlet RV | 6 (8) | Labcor | 4 (5) |
| II | 32 (41) | PA with intact septum | 6 (8) | Others | 2 (2.5) |
| III | 6 (8) | Truncus arteriosus | 5 (6) | ||
| No. of previous operations | TGA (Jatene) | 3 (4) | |||
| 1 | 28 (36) | Others | 2 (3) | ||
| 2 | 24 (31) | ||||
| ≥ 3 | 26 (33) | ||||
| Type of RVOT lesion | |||||
| Mixed | 40 (51) | ||||
| PS | 22 (28) | ||||
| PR | 16 (21) | ||||
AoS, aortic stenosis; NYHA, New York Heart Association; PA, pulmonary atresia; PR, pulmonary regurgitation; PS, pulmonary stenosis; RV, right ventricle; RVOT, right ventricular outflow tract; Su, surgical; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Data are expressed as No. (%) or median [interquartile range].
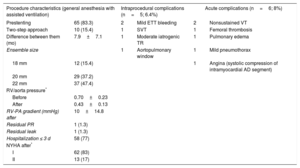
Procedure Characteristics of 78 Primary Percutaneous Pulmonary Valve Implantations
| Procedure characteristics (general anesthesia with assisted ventilation) | Intraprocedural complications (n=5; 6.4%) | Acute complications (n=6; 8%) | |||
|---|---|---|---|---|---|
| Prestenting | 65 (83.3) | 2 | Mild ETT bleeding | 2 | Nonsustained VT |
| Two-step approach | 10 (15.4) | 1 | SVT | 1 | Femoral thrombosis |
| Difference between them (mo) | 7.9±7.1 | 1 | Moderate iatrogenic TR | 1 | Pulmonary edema |
| Ensemble size | 1 | Aortopulmonary window | 1 | Mild pneumothorax | |
| 18 mm | 12 (15.4) | 1 | Angina (systolic compression of intramyocardial AD segment) | ||
| 20 mm | 29 (37.2) | ||||
| 22 mm | 37 (47.4) | ||||
| RV/aorta pressure* | |||||
| Before | 0.70±0.23 | ||||
| After | 0.43±0.13 | ||||
| RV-PA gradient (mmHg) after | 10±14.8 | ||||
| Residual PR | 1 (1.3) | ||||
| Residual leak | 1 (1.3) | ||||
| Hospitalization ≤ 3 d | 58 (77) | ||||
| NYHA after* | |||||
| I | 62 (83) | ||||
| II | 13 (17) | ||||
AD, anterior descending coronary artery; ETT, endotracheal tube; NYHA, New York Heart Association; PA, pulmonary artery; PR, pulmonary regurgitation; RV, right ventricle; SVT, supraventricular tachycardia; TR, tricuspid regurgitation; VT, ventricular tachycardia.
Values represent No. (%) or mean±standard deviation.
In terms of periprocedural complications, there were no incidences of coronary compression or death. The most serious intraprocedural complication was an iatrogenic aortopulmonary window in 1 patient undergoing Jatene surgery (11 years old, 66kg), which was initially resolved using percutaneous implantation of an 11-mm muscular ventricular septal defect closure device with subsequent elective surgical closure of the residual shunt. The remainder of the complications were mild (Table 3) and were resolved with conservative treatment. In the first postimplantation hours, one 16-year-old patient with tetralogy of Fallot had transient angina not related to mechanical interference of the prosthesis with the origin of the coronary arteries (evaluated using computed tomography and angiography), but to systolic compression of the intramyocardial coronary segment of the anterior descending artery; the situation was resolved using beta-blocker therapy.
In total, because 3 patients moved to their area of origin (which was different from that of the implanting hospital), 75 patients completed follow-up. The median follow-up time was 2.4 [1.1-4.9] years. In the final follow-up echocardiography, the mean peak pulmonary valve gradient was 25.0±26.5mmHg, 3 patients (4%) had > grade II pulmonary regurgitation, and 1 (1.3%) had mild perivalvular leak.
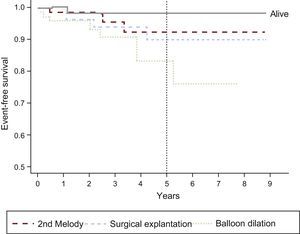
Thirteen patients required some type of reintervention: 8 balloon overdilations (3 without prestenting of the RVOT), 5 explantations (3 due to infectious endocarditis [IE] and 2 due to loss of stent integrity caused by fracture and/or sternal compression), and 2 valve-in-valve implantations (due to valve dysfunction secondary to fracture). Accordingly, 97%±2%, 95%±3%, and 80%±7% of patients were reintervention-free (ie, balloon overdilation, valve-in-valve implantation, or explantation) at 1, 2, and 5 years, respectively. There were no significant differences between patients in terms of weight (reference, 30kg), anatomical substrate, or type of preimplantation RVOT lesion. The event-free survival times for the various events are shown in Figure 2. After 1, 2, and 5 years of follow-up, 97%±2%, 93%±3.5%, and 83%±6% of patients were pulmonary valve replacement-free (percutaneous or surgical), respectively (Figure 3). There was 1 death (1.3%), related to IE.
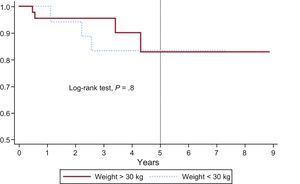
Pulmonary valve replacement-free survival curve (percutaneous or surgical) in patients weighing more than 30kg and less than 30kg. At 1, 2, and 5 years of follow-up, there was no need for valve replacement in a mean±standard error of 100%, 95%±0.05%, and 84%±0.09% in patients weighing less than 30kg vs 96%±0.03%, 96%±0.03%, and 83%±0.09% in those weighing more than 30kg. Differences were not statistically significant.
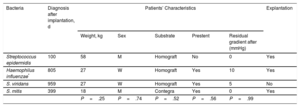
There were 4 cases of IE (affecting 5.6% of the total population) (Table 4): 1 occurring at an early stage in a patient with a history of preimplant endocarditis with the same bacterium; 3 patients required surgical extraction of the valve, performed 9, 19, and 78 days after diagnosis (corresponding to 814, 418, and 178 days after implantation). At the 5-year follow-up, 92% of patients were disease-free, giving a mean IE incidence of 1.8% per patient-year.
Description of Patients Diagnosed With Infectious Endocarditis
| Bacteria | Diagnosis after implantation, d | Patients’ Characteristics | Explantation | ||||
|---|---|---|---|---|---|---|---|
| Weight, kg | Sex | Substrate | Prestent | Residual gradient after (mmHg) | |||
| Streptococcus epidermidis | 100 | 58 | M | Homograft | No | 0 | Yes |
| Haemophilus influenzae* | 805 | 27 | W | Homograft | Yes | 10 | Yes |
| S. viridans | 959 | 27 | W | Homograft | Yes | 5 | No |
| S. mitis | 399 | 18 | M | Contegra | Yes | 0 | Yes |
| P=.25 | P=.74 | P=.52 | P=.56 | P=.99 | |||
M, man; W, woman.
The durability of surgical RVOT interventions for complex congenital heart diseases varies considerably according to patient age and the type of material used.5,6 One of the studies with the largest sample size reported a mean intervention-free survival of 11.2 years for adult-sized conduits (> 18mm).7 Right ventricular outflow tract dysfunction occurs without warning over time due to diverse mechanisms: pulmonary regurgitation, somatic growth, stenotic anastomosis, valvular stenosis, conduit kinking, sternal compression, intimal proliferation, calcification, and aneurysmatic dilatation.8 Right ventricular outflow tract reconstruction can be performed with low mortality but can be associated with high morbidity, particularly given the need for surgical reinterventions. The strategy of choice for PPVI is to minimize reinterventions without compromising ventricular volume or function. The main controversial aspects are the time of RVOT valve placement and the choice between PPVI and surgery. Given the intrinsic complexity of congenital heart disease, due to its rarity and variability, no studies have directly compared PPVI with surgery, which is why descriptive multicenter studies are the main source of information available.
According to the 2010 European guidelines, in general, the following patients should be treated: symptomatic patients with a RV pressure > 60mmHg and/or moderate/severe pulmonary regurgitation, asymptomatic patients with pulmonary stenosis or severe pulmonary regurgitation with impaired exercise capacity or progressive dilation or systolic dysfunction of the RV, progressive tricuspid regurgitation, pulmonary gradient > 80mmHg, or sustained atrial or ventricular tachycardias.9 In 2011, the American Heart Association recommended (IIa B) that PPVI be considered in patients with a conduit between the RV and the pulmonary artery with moderate/severe pulmonary stenosis or pulmonary regurgitation, according to the inclusion/exclusion criteria of each valve.10
Several countries have now published national prospective registries of the Melody PPVI, with excellent short- and mid-term results (Table 5).4,11–15 The procedure has been considered successful (Melody implanted in the desired location, mild or minor residual pulmonary regurgitation, an RV-pulmonary artery gradient < 35mmHg, absence of explantation 24hours after the procedure)16 in 93% to 99% of cases.4,14,17 As in our study, there was a significant reduction in the RV/aorta pressure ratio4,12–15 and a general improvement in hemodynamic parameters17 and New York Heart Association functional class.13,14,16 However, our series is the first to specifically analyze the results obtained in a pediatric population, including those weighing less than 20 to 30kg and with various anatomical substrates for the implant.
Characteristics of the Main Registries of Melody Percutaneous Pulmonary Valve Implantation: Prospective and Multicenter Observational Studies
| Publication | Patients, n | Age, y | Weight, kg | Fallot/PA+VSD | RVOT lesion indication | RV-PA conduit | Prestent | Follow-up time | Hospitalization time, d | Complications | Death | Endocarditis | Explantation | Balloon dilatation | Valve-in-valve |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011, Germany Eicken et al.11 | 102 | 21.5 [16.2-30.1] | 63 [54.2-75.9] | 61 (60) | Not described | 100% (79% homograft) | 96 (94) | 352 [99-390] d | 2 | 2 major (2%) 1 LCA compression 1 transient complete AVB | 1 (1%) | 1 (1) Staphylococcus aureus | 1 (1) | 8 (7.8) | 4 (3.4) |
| 2013, Italy Butera et al.4 | 63 (implantations, 61) | 24 [11-65] | 60 [30-140] | 39 (62) | 30 Mixed 21 PS 12 PR | 100% (33% homograft, 28% bioprosthesis) | 53 (87) | 2.4 y | 5 [3-45] | 9 major+minor (14%) 1 valve embolization 1 VF 3 vascular access 1 mild ETT bleeding 2 transfusions 1 conduit tear | 4 (6.5%) | 2 (3.3) Staphylococcus aureus | 2 (3.3) | Not described | 2 (3.3) |
| 2014, France Fraisse et al.12 | 64 | 21.4 [10.5-77.3] | Not described > 25 kg | 32 (50) | 67% Mixed 21.9% PS 12.9% PR | 100% (subtypes not described) | 62 (97) | 4,6 [0.2-5.2] y | 3 [2-5] | Minor (17%) 7 homograft tears 1 ETT bleeding 1 false femoral artery aneurysm 1 inguinal hematoma 1 transfusion for access site bleeding | 3 (4.5%) 2 IE 1 CHF | 4 (6.3) Staphilococcus epidermidis Streptococcus sanguinis Staphilococcus haemolyticus | 3 (4.7) | Not described | 0 |
| 2015, United States Cheatham et al.13 (2010, McElhinney et al.,14 Zahn et al.15) | 150 (implantations, 136) | 19 [7-53] | Not described > 25 kg | 77 (51) | 80 (53) PR 39 (26) PS 31 (21) Mixed | 100% (74% homograft, 21% bioprosthesis) | 49 (36) | 4,5 [0.4-7] y | 1-7 | 8 major+minor (6%) 1 conduit rupture 1 coronary artery dissection 1 conduit tear 1 SVT (cardioversion) 1 hypercapnia 1 femoral thrombosis 2 guidewire perforations of lobar artery | 5 (3.7) | 14 (10.3) (unknown) | 11 (8.1) | 6 (4.4) | 20 (14.7) |
AVB, atrioventricular block; CHF, congestive heart failure; ETT, endotracheal tube; IE, infectious endocarditis; LCA, left coronary artery; PA, pulmonary artery; PR, pulmonary regurgitation; PS, pulmonary stenosis; RV, right ventricle; RVOT, right ventricular outflow tract; SVT, sustained ventricular tachycardia; VF, ventricular fibrillation; VSD, ventricular septal defect.
Unless otherwise indicated, the data represent No. (%) or median [interquartile range].
The overall reported rate of periprocedural complications is about 6% with a 2.7% rate of major complications (conduit rupture, pulmonary artery perforation, tricuspid valve injury, valve dislodgement, coronary artery compression, or pulmonary artery obstruction) and a 11.9% rate of minor complications.16 Our series shows rates of acute major complications of 2.6% (n=2, aortopulmonary window and tricuspid valve injury) and of minor complications of 3.8%. Prediction of coronary compression risk is one of the most difficult steps in the procedure and this complication is the event most frequently associated with death in the catheterization laboratory. An estimated 5% to 6% of all possible PPVI candidates have coronary anatomy that is susceptible to compression during implantation.4,11 Additionally, there is greater risk of RVOT rupture/tear in highly calcified conduits and homografts.18 Fortunately, none of these events occurred in our patients.
During the short- and mid-term follow-up, the overall mortality ranged from 0% to 5% and it was generally not related to the device,16 except in severe cases of IE. The most frequent cause of percutaneous or surgical reintervention was reestenosis of the valve stent, caused by late recoil or by loss of radial force secondary to fracture.16 The risk factors for the development of fractures are younger age, a significant pre- and postprocedural RV-pulmonary artery gradient, lower conduit diameter on angiography, stent recoil or graft compression, and valve position directly under the sternum.19,20 Hence, the importance of RVOT conditioning with prestenting before the implantation. The development of significant pulmonary regurgitation due to injury to the valve cusps is a rare condition typically associated with IE.21,22 Generally, the valve dysfunction- or reintervention-free survival exceeds 90%, 80%, and 70% at 1, 2, and 4 years, respectively,14,19,23 as seen in our study. Specifically, the US Investigational Device Exemption Trial reported 5-year reintervention- and explantation-free survivals of 76%±4% and 92%±3%, respectively. The factors associated with reintervention are a high residual gradient (mean gradient > 25mmHg)13,23 or stent compression/recoil immediately after valve release.18 On the other hand, protective factors are prestenting,13,18 which stabilizes the RVOT, and operator experience.23 Prestenting can be performed in the same procedure or 2 or 3 months beforehand to allow endothelial growth and better stent fixation.24 Retrospective analysis of a national surgical pulmonary valve implantation series25 showed a 5-year replacement-free survival due to prosthesis dysfunction in patients younger than 20 years of about 90% (although our series includes the initial experience with PPVI without prestenting for RVOT conditioning in 7 patients).
The mid-term risk of IE has been estimated at 2.4% per patient-year,21 although more than half of infections do not directly affect the valve cusps and most respond to antibiotic therapy. Regardless, IE is the main cause of surgical explantation and death due to sepsis.4,11,21 The possible pathophysiological mechanisms involved include turbulence caused by high residual gradient, in situ thrombosis, and poor adherence to prophylactic antibiotics.4,26 This risk is not exclusive to PPVI; an example is given by the series of Oliver et al.,25 with a postoperative IE incidence of 11.5% during a median follow-up of 7 years. Bovine jugular tissue is generally associated with greater infectious risk than other biological or synthetic prosthetic material.27,28 A recent in vitro study reported that its manipulation during percutaneous procedures can cause histological changes and microfractures.29 Accordingly, these patients should be treated with lifelong antiplatelet therapy with aspirin and undergo a dental evaluation before the PPVI. Our series showed an IE incidence slightly lower than that seen in the literature, undoubtedly related to the lower weight of our patients (fewer dental and dermatologic problems) and because 19% of the native RVOTs were conduit-free.
Resolution of RVOT dysfunction via PPVI has also been associated with reverse remodeling of the RV,11,12,14,30 better left ventricular filling,30 and even improved tricuspid regurgitation.31
Percutaneous pulmonary valve implantation also modifies the ventricular electrophysiological substrate32 and, in the acute postimplantation period, new arrhythmias develop in 7% to 15% of patients, generally extrasystole or nonsustained ventricular tachycardia.32,33 Such arrhythmias are considered transient phenomena that spontaneously resolve, generally in the first 6 months, particularly in smaller patients and those requiring conservative treatment (and beta-blockers should be tried). Another possibly associated mechanism would be placement of the endoprosthesis so that it touches the RVOT muscle. In our series, 2.6% of patients had episodes of nonsustained ventricular tachycardia during the acute postprocedural period; these patients were treated with beta-blockers for 12 to 18 months and had a favorable course.
As mentioned above, PPVI is officially approved for patients weighing more than 30kg with dysfunctional surgical RVOT conduits with diameters of 18 to 22mm for Melody valves and 23 to 26mm for SAPIEN valves (Edwards Lifescience LLC; California, United States). However, it is estimated that just 20% of patients with congenital heart disease and RVOT dysfunction meet these criteria. Thus, most patients would be candidates for off-label PPVI.2 In our series, such patients comprised 47.4% (37 of 78), due to placement in a different anatomical substrate of the conduit (n=23) and/or due to weight < 30kg (n=22).
Few articles discuss PPVI in patients younger than 5 years, given that the technique is limited by the high profile of the sheath (22-24 Fr). Good short-term hemodynamic results were obtained in a series of 25 patients published in 2010 (median age, 8 years; median weight, 21.4kg).34 The incidence of major complications was 16% but the only complication associated with lower weight was abdominal hematoma development. Thus, femoral venography is recommended if access is obtained at this site, as well as use of jugular access for patients weighing less than 20kg, to obtain a favorable RV curvature and to not overdilate the conduit more than 110% of the initial size. During a median follow-up of 16 months in 22 patients, there was 1 surgical conduit replacement, 2 fractures treated with valve-in-valve PPVI, and 2 cases of endocarditis. Other authors theorize that younger patients benefit most from the hemodynamic effects of PPVI in terms of reverse remodeling of the RV and maximal oxygen consumption.35
In addition, various studies have shown good hemodynamic results for the Melody PPVI over native or patch-extended RVOT36,37 and over surgical bioprosthetic valves.38 In the case of the latter, the 2-year reintervention-free survival was higher than 90% and there were no procedure-related deaths.
Good results have also been obtained after implantation of a second Melody valve by the valve-in-valve technique, as long as there were no external obstacles impeding correct valve expansion (eg, sternal compression).39
CONCLUSIONSThe results of Melody PPVI in pediatric patients in Spain show that it is a viable, successful, and safe technique, even in those weighing less than 30kg or with native RVOT. The short- and mid-term hemodynamic results are excellent and there is low morbidity and mortality. The incidence of IE was relatively low, possibly related to the younger age and absence of conduits in many of our patients. We consider our work to be a gateway to new prospective studies involving interhospital collaboration that include adult patients and shed light on the ventricular remodeling data and life expectancy of these patients.
CONFLICTS OF INTERESTJ.L. Zunzunegui is a proctor for the Medtronic percutaneous pulmonary valve implant.
- –
The classic treatment strategy for RVOT dysfunction, established 4 decades ago, has been interposition of surgical prosthetic material between the ventricle and pulmonary artery. During their clinical course, the treated patients required frequent surgical replacements due to growth and degeneration of this material over time. Subsequent advances with the arrival of percutaneous treatment have progressed from high-pressure balloon angioplasty and stent placement to PPVI. Numerous studies now show that PPVI is viable and achieves excellent results.
- –
Our study shows the national experience with the Melody PPVI in pediatric patients, focusing on valve functioning during follow-up and the possible development of complications, particularly endocarditis.
Cardiac Catherization Working Group of the Spanish Society of Pediatric Cardiology and Congenital Heart Disease.