The clinical benefits of optical coherence tomography-guided percutaneous coronary intervention are unclear. Therefore, in this study we sought to evaluate the impact of optical coherence tomography guidance on stent strut coverage following drug-eluting stent implantation.
MethodsA total of 101 patients in 105 lesions were randomly assigned to receive percutaneous coronary intervention under either optical coherence tomography guidance (n = 51 lesions of 50 patients) or angiography guidance (n = 54 lesions of 51 patients), and underwent a follow-up optical coherence tomography examination 6 months after zotarolimus-eluting stent implantation. The primary and secondary end points were the percentage of uncovered and malapposed struts, respectively, on 6-month follow-up optical coherence tomography.
ResultsThe percentage of uncovered struts was significantly lower in the optical coherence tomography-guided arm (1.60% [1.84]%, [median, 1.06%] vs 4.51% [5.43]% [median, 2.38%]; P = .0004) at 6-month follow-up. The incidence of stents with ≥ 5.9% uncovered struts was also significantly lower in the optical coherence tomography-guided arm (2 patients [3.9%] vs 14 patients [25.9%]; P = .002). In addition, the percentage of malapposed struts was significantly lower in the optical coherence tomography-guided arm (0.19% [0.51]% [median, 0.0%] vs 0.98% [2.53]% [median, 0.0%]; P = .027).
ConclusionsOptical coherence tomography-guided percutaneous coronary intervention significantly reduced the incidence of uncovered stent struts at 6 months compared to angiography-guided percutaneous coronary intervention. These findings suggest that optical coherence tomography-guided percutaneous coronary intervention has a beneficial effect on drug-eluting stent strut coverage.
Keywords
Delayed vascular and endothelial healing is associated with stent thrombosis following implantation of a drug-eluting stent (DES) in pathologic studies.1,2 High-resolution assessment of stent struts in vivo3 using optical coherence tomography (OCT) shows that uncovered stent struts are associated with late stent thrombosis after DES implantation.4,5 Previous studies show that larger-sized acute stent malapposition may be responsible for persistent stent malapposition and the development of uncovered stent struts during follow-up.6,7 Compared to intravascular ultrasound (IVUS), high-resolution OCT results in superior apposition of stent struts to the vessel wall. To determine if this improved strut apposition was associated with improved stent strut coverage, we performed a prospective randomized study comparing strut coverage during follow-up after either OCT-guided or angiography-guided DES implantation.
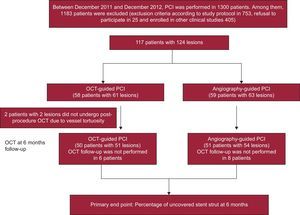
METHODSStudy PopulationThis study was a prospective, open-label, randomized, single-center trial, registered at ClinicalTrials.gov (NCT01869842). A total of 117 patients with 124 coronary lesions were enrolled between December 2011 and December 2012 and randomly assigned to receive either OCT- or angiography-guided implantation of a zotarolimus-eluting stent (Endeavor ResoluteTM, Medtronic CardioVascular; Santa Rosa, California, United States). Inclusion criteria were: a) age ≥ 20 years old and significant coronary de novo lesion(s) (≥ 70% diameter stenosis on visual estimation), and b) a native coronary artery with a reference vessel diameter between 2.5mm and 4.0mm that could be covered by a single stent. Exclusion criteria were the following: a) refusal to participate; b) participation in other study protocols; c) lesions with significant left main disease or chronic total occlusion; d) lesions in a grafted vessel, thrombosis, or bifurcation lesions requiring 2 stents; e) an ejection fraction ≤ 30%; f) allergy to either antiplatelet agents or the contrast dye; g) known renal failure with baseline creatinine level ≥ 2.0mg/dL, or end-stage renal disease; h) life expectancy < 1 year; i) prior DES treatment of a different vessel within 3 months; j) presence of an overlapping stent or long stent (> 3 0mm); k) lesion calcification visible on angiography, and l) current pregnancy, or women of childbearing potential. During the same study period, percutaneous coronary intervention (PCI) was performed in 1300 patients. Of these patients, 1183 were excluded. Twenty-five patients refused participation, 405 were participating in other study protocols, and 753 met the remaining criteria, as follows: left main disease in 75, chronic total occlusion in 55, graft vessel disease in 23, totally occluded thrombotic lesion in 125, bifurcation lesions requiring 2 stents in 45, ejection fraction < 30% in 48, chronic renal failure (creatinine level ≥ 2mg/dL) in 92, long lesion (> 30mm stent) or overlapping stents in 135, and anatomy not suitable for OCT procedure in 155. This randomized study was approved by the institutional review board of our institute and written consent was obtained from all enrolled patients.
Randomization and Study ProceduresAll study participants fulfilling the enrollment criteria of this study were randomly assigned in a 1:1 ratio by an interactive web-based response system to receive OCT- or angiography-guided PCI. To preserve a balance between the 2 strategies, randomization was stratified according to the presence of diabetes mellitus, acute coronary syndrome, and the estimated length and diameter of the prospective DES implant. All patients received at least 75mg of acetylsalicylic acid and a loading dose of 300mg of clopidogrel at least 12hours pre-PCI. Unfractionated heparin was administered as needed to maintain the activated clotting time of > 250seconds. All PCI procedures were performed according to current standard techniques. In the OCT-guided arm, adjuvant postdilation was performed at operator discretion based on OCT findings. In the angiography-guided arm, stent optimization including adjuvant postdilation was based on a visual estimation of angiographic findings and procedural success was defined as ≤ 20% residual stenosis after stent placement by visual estimation. Postprocedure treatment included a 12-month prescription of dual antiplatelet therapy with 100mg acetylsalicylic acid and 75mg clopidogrel daily.
Quantitative Coronary Angiography AnalysisQuantitative coronary angiography analysis was performed before and after stent implantation, and at 6-month follow-up using an off-line quantitative coronary angiographic system (CASS system, Pie Medical Instruments; Maastricht, The Netherlands) in an independent core laboratory (Cardiovascular Research Center, Seoul, Korea). Reference vessel and minimal luminal diameters were obtained by comparison to the guidance catheter from diastolic frames in a single, matched view showing the smallest minimal luminal diameter: post-PCI and follow-up angiograms were evaluated in the same projection. Acute gain was defined as the difference between preprocedure and postprocedure minimal luminal diameter. Late loss was defined as the change in minimal luminal diameter between postprocedure and follow-up.
Optical Coherence Tomography Imaging and AnalysisAt postprocedure and 6-month follow-up after PCI, OCT imaging of the target lesion was performed using a frequency–domain OCT system (C7-XR OCT imaging system, LightLab Imaging, Inc., St. Jude Medical; St. Paul, Minnesota, United States). In this study, OCT cross-sectional images were recorded at 100 fps while a catheter was pulled back at 20mm/s within the stationary imaging sheath. All OCT images were analyzed blind by independent analysts at the core laboratory (Cardiovascular Research Center).
Cross-sectional OCT images were analyzed at 1mm intervals. Stent and luminal cross-sectional areas were measured, and neointimal hyperplasia cross-sectional area was assessed as the difference between the stent and luminal cross-sectional areas. Mean or median values are reported in this study. The neointimal hyperplasia thickness was measured as the distance between the endoluminal surface of the neointima and the strut, and an uncovered strut was defined as having a neointimal hyperplasia thickness of 0μm.8 A malapposed strut of zotarolimus-eluting stent was defined as detachment from the vessel wall by ≥ 110μm (stent strut thickness 91μm + abluminal polymer thickness 6μm, considering blooming artifact). The proportions of uncovered or malapposed struts were identified from OCT cross-sections, and are expressed as a percentage of total struts visible in the survey. In addition, uncovered struts associated with major adverse cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and stent thrombosis, were subclassified based on the degree of strut coverage as described (favorable coverage, < 5.9% uncovered; unfavorable coverage, ≥ 5.9% uncovered).9 Malapposed struts, which included any amount of malapposition, were further classified into persistent, resolved, or late stent malapposition, according to the change in malapposition visible in matching frames between postprocedure and follow-up OCT.10 Intrastent thrombi were defined as irregular masses protruding into the lumen, > 250μm at the thickest point.11
Study End Points and Clinical Follow-upClinical follow-up was performed at 1, 3, 6, and 12 months after PCI; angiographic and OCT follow-up was performed at 6 months. The primary end point was the percentage of uncovered struts in the 6-month follow-up OCT assessments. Secondary end points were: a) the presence of malapposed struts in the 6-month follow-up OCT; b) the occurrence of major adverse cardiac events at 12 months, defined as a composite of cardiac death, nonfatal myocardial infarction, or patients requiring target lesion revascularization, and c) stent thrombosis at 12 months, as defined by the Academic Research Consortium.12
Statistical AnalysisThis study was designed to compare the 6-month follow-up strut coverage of OCT-guided PCI vs angiography-guided PCI. We hypothesized that the improved stent apposition associated with high-resolution OCT guidance might reduce the incidence of uncovered struts by 50% at the 6-month follow-up OCT, compared to angiography guidance. Assuming 4.8% (4.3%) incidence of uncovered struts at 6-month follow-up, based on our prior studies of zotarolimus-eluting stents at 3 months (6.2%) and 9 months (3.4%),13,14 a sample size of 51 patients for each arm would provide 80% power and a 5% alpha error rate. Taking into account a 10% loss rate and exclusion due to poor OCT image quality, 114 patients were needed for the study. Statistical analysis was performed with the Statistical Analysis System software (SAS; 9.1.3., SAS Institute; North Carolina, United States). Categorical data are presented as numbers and percentages, and were compared with chi-square statistics or Fisher exact test. Continuous data are presented as the mean (standard deviation) (median) and compared with the Student t test. If the distributions were skewed, a nonparametric test was used. Hierarchical linear modeling was applied for the clustering problem. Specifically, data for lesions, cross-sections, and struts were modeled for each patient as a random effect variable. With this modeling, the clustering and correlation problems could be controlled. In case of clustered variables with nonnormal distributions, we integrated the results of hierarchical linear modeling with nonparametric analysis. A value of P < .05 denoted statistical significance.
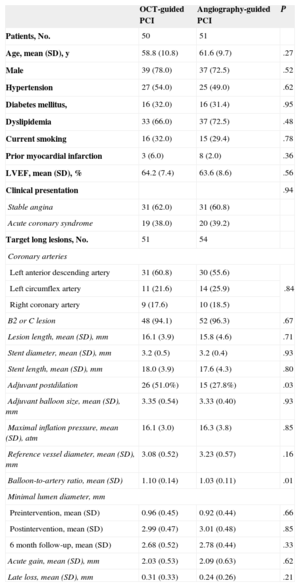
RESULTSA total of 117 patients with 124 coronary lesions were randomly assigned into treatment groups receiving PCI with either OCT guidance (n = 58) or angiography guidance (n = 59). Postprocedure OCT was performed on 56 patients (59 lesions) in the OCT-guided arm and revealed that the OCT catheter had not passed through the lesions in 2 patients (2 lesions). In the OCT-guided arm, 50 patients (51 lesions) received follow-up at 6 months, compared to 51 patients (54 lesions) in the angiography-guided arm; 10 patients refused follow-up coronary angiography; 1 patient had in-stent restenosis with tight narrowing; 1 patient had subacute stent thrombosis; the OCT catheter was not passed through the stented lesions in 2 patients. The overall study diagram is shown in Figure 1. Baseline patient characteristics were comparable between the 2 arms (Table 1). Adjuvant postdilation was more frequent in the OCT-guided arm (51.0% vs 27.8%, P = .03). However, additional treatment was not performed in cases of OCT-detected small-sized thrombus or tissue prolapse and minor edge dissection. In total, 101 patients (105 lesions) were evaluated by OCT at follow-up, and there were no serious complications during the procedure. Serial OCT findings are summarized in Table 2.
Patient Characteristics
| OCT-guided PCI | Angiography-guided PCI | P | |
|---|---|---|---|
| Patients, No. | 50 | 51 | |
| Age, mean (SD), y | 58.8 (10.8) | 61.6 (9.7) | .27 |
| Male | 39 (78.0) | 37 (72.5) | .52 |
| Hypertension | 27 (54.0) | 25 (49.0) | .62 |
| Diabetes mellitus, | 16 (32.0) | 16 (31.4) | .95 |
| Dyslipidemia | 33 (66.0) | 37 (72.5) | .48 |
| Current smoking | 16 (32.0) | 15 (29.4) | .78 |
| Prior myocardial infarction | 3 (6.0) | 8 (2.0) | .36 |
| LVEF, mean (SD), % | 64.2 (7.4) | 63.6 (8.6) | .56 |
| Clinical presentation | .94 | ||
| Stable angina | 31 (62.0) | 31 (60.8) | |
| Acute coronary syndrome | 19 (38.0) | 20 (39.2) | |
| Target long lesions, No. | 51 | 54 | |
| Coronary arteries | |||
| Left anterior descending artery | 31 (60.8) | 30 (55.6) | .84 |
| Left circumflex artery | 11 (21.6) | 14 (25.9) | |
| Right coronary artery | 9 (17.6) | 10 (18.5) | |
| B2 or C lesion | 48 (94.1) | 52 (96.3) | .67 |
| Lesion length, mean (SD), mm | 16.1 (3.9) | 15.8 (4.6) | .71 |
| Stent diameter, mean (SD), mm | 3.2 (0.5) | 3.2 (0.4) | .93 |
| Stent length, mean (SD), mm | 18.0 (3.9) | 17.6 (4.3) | .80 |
| Adjuvant postdilation | 26 (51.0%) | 15 (27.8%) | .03 |
| Adjuvant balloon size, mean (SD), mm | 3.35 (0.54) | 3.33 (0.40) | .93 |
| Maximal inflation pressure, mean (SD), atm | 16.1 (3.0) | 16.3 (3.8) | .85 |
| Reference vessel diameter, mean (SD), mm | 3.08 (0.52) | 3.23 (0.57) | .16 |
| Balloon-to-artery ratio, mean (SD) | 1.10 (0.14) | 1.03 (0.11) | .01 |
| Minimal lumen diameter, mm | |||
| Preintervention, mean (SD) | 0.96 (0.45) | 0.92 (0.44) | .66 |
| Postintervention, mean (SD) | 2.99 (0.47) | 3.01 (0.48) | .85 |
| 6 month follow-up, mean (SD) | 2.68 (0.52) | 2.78 (0.44) | .33 |
| Acute gain, mean (SD), mm | 2.03 (0.53) | 2.09 (0.63) | .62 |
| Late loss, mean (SD), mm | 0.31 (0.33) | 0.24 (0.26) | .21 |
LVEF, left ventricular ejection fraction; OCT, optical coherence tomography. PCI, percutaneous coronary intervention; SD, standard deviation.
Unless otherwise indicated, data are expressed as no. (%) or mean (standard deviation).
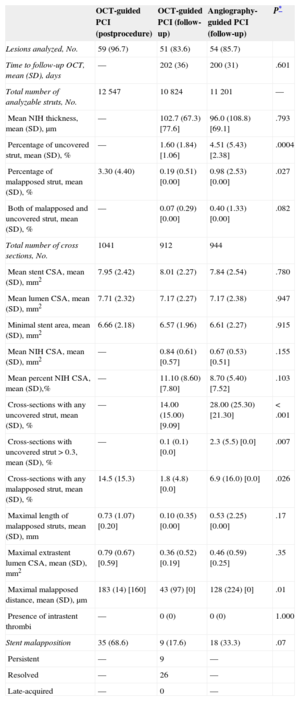
Optical Coherence Tomography Assessment of Stents at Immediate Postprocedure and 6-month Follow-up
| OCT-guided PCI (postprocedure) | OCT-guided PCI (follow-up) | Angiography-guided PCI (follow-up) | P* | |
|---|---|---|---|---|
| Lesions analyzed, No. | 59 (96.7) | 51 (83.6) | 54 (85.7) | |
| Time to follow-up OCT, mean (SD), days | — | 202 (36) | 200 (31) | .601 |
| Total number of analyzable struts, No. | 12 547 | 10 824 | 11 201 | — |
| Mean NIH thickness, mean (SD), μm | — | 102.7 (67.3) [77.6] | 96.0 (108.8) [69.1] | .793 |
| Percentage of uncovered strut, mean (SD), % | — | 1.60 (1.84) [1.06] | 4.51 (5.43) [2.38] | .0004 |
| Percentage of malapposed strut, mean (SD), % | 3.30 (4.40) | 0.19 (0.51) [0.00] | 0.98 (2.53) [0.00] | .027 |
| Both of malapposed and uncovered strut, mean (SD), % | — | 0.07 (0.29) [0.00] | 0.40 (1.33) [0.00] | .082 |
| Total number of cross sections, No. | 1041 | 912 | 944 | |
| Mean stent CSA, mean (SD), mm2 | 7.95 (2.42) | 8.01 (2.27) | 7.84 (2.54) | .780 |
| Mean lumen CSA, mean (SD), mm2 | 7.71 (2.32) | 7.17 (2.27) | 7.17 (2.38) | .947 |
| Minimal stent area, mean (SD), mm2 | 6.66 (2.18) | 6.57 (1.96) | 6.61 (2.27) | .915 |
| Mean NIH CSA, mean (SD), mm2 | — | 0.84 (0.61) [0.57] | 0.67 (0.53) [0.51] | .155 |
| Mean percent NIH CSA, mean (SD),% | — | 11.10 (8.60) [7.80] | 8.70 (5.40) [7.52] | .103 |
| Cross-sections with any uncovered strut, mean (SD), % | — | 14.00 (15.00) [9.09] | 28.00 (25.30) [21.30] | < .001 |
| Cross-sections with uncovered strut > 0.3, mean (SD), % | — | 0.1 (0.1) [0.0] | 2.3 (5.5) [0.0] | .007 |
| Cross-sections with any malapposed strut, mean (SD), % | 14.5 (15.3) | 1.8 (4.8) [0.0] | 6.9 (16.0) [0.0] | .026 |
| Maximal length of malapposed struts, mean (SD), mm | 0.73 (1.07) [0.20] | 0.10 (0.35) [0.00] | 0.53 (2.25) [0.00] | .17 |
| Maximal extrastent lumen CSA, mean (SD), mm2 | 0.79 (0.67) [0.59] | 0.36 (0.52) [0.19] | 0.46 (0.59) [0.25] | .35 |
| Maximal malapposed distance, mean (SD), μm | 183 (14) [160] | 43 (97) [0] | 128 (224) [0] | .01 |
| Presence of intrastent thrombi | — | 0 (0) | 0 (0) | 1.000 |
| Stent malapposition | 35 (68.6) | 9 (17.6) | 18 (33.3) | .07 |
| Persistent | — | 9 | — | |
| Resolved | — | 26 | — | |
| Late-acquired | — | 0 | — |
CSA, cross-sectional area; NIH, neointimal hyperplasia; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Unless otherwise indicated, data are expressed as No. (%), mean (standard deviation) or [median].
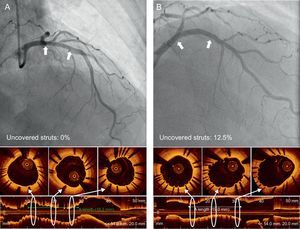
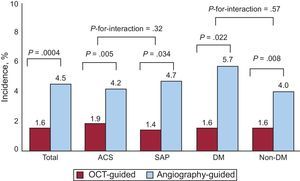
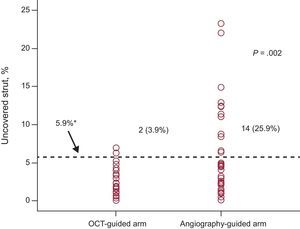
In the OCT-guided arm, the incidences of malapposed struts and lesions with any malapposed strut were 3.3% and 66. 1% (39 of 59), respectively, immediately after the index procedure. The percentage of uncovered struts at 6 months was significantly lower using OCT-guidance (1.60% [1.84]% [median, 1.06%] vs 4.51% [5.43]% [median, 2.38%] using angiography; P = .0004). Figure 2 shows representative examples of follow-up angiography and OCT between OCT- and angiography-guided PCI. Angiography cannot detect strut coverage. Moreover, stent strut coverage was more favorable in OCT guidance irrespective of clinical presentation or the presence of diabetes mellitus (Figure 3). At the 6-month follow-up OCT, the incidence of unfavorable strut coverage (≥ 5.9% of uncovered struts) was significantly lower in the OCT-guided arm [2 patients (3.9%) vs 14 patients (25.9%), P = .002; Figure 4], as was the percentage of malapposed struts (0.19% [0.51]% [median, 0.0%] vs 0.98% [2.53]% [median, 0.0%]; P = .027).
The incidence and severity of uncovered stent struts. The occurrence and severity of stents with unfavorable strut coverage (≥ 5.9%) was significantly lower in optical coherence tomography guidance than angiography guidance. OCT, optical coherence tomography.
*The cutoff value of 5.9% is related with clinical outcomes based on previous study.9
One-year clinical follow-up was completed in all enrolled patients. There were no significant differences in major adverse cardiac events (2 of 58 [3.4%] in the OCT arm vs 3 of 59 [5.1%] in the angiography arm). Stent thrombosis occurred in a single patient in the angiography arm 2 days after implantation; target lesion revascularization was performed in 4 patients (2 OCT-guided patients and 2 angiography-guided patients). Of these 5 patients, 6-month follow-up OCT evaluation was not performed on the patient with stent thrombosis, and we were unable to pass the OCT catheter through the tightest lesion in 1 of the OCT-guided patients who underwent target lesion revascularization.
DISCUSSIONOur randomized, prospective comparison of OCT- and angiography-guided PCI demonstrated that OCT-guidance was associated with significantly improved outcomes with respect to strut coverage and stent apposition at 6 months. In addition, the incidence of stents with unfavorable strut coverage (≥ 5.9% of uncovered struts) was significantly lower following OCT guidance compared to angiography guidance. Adjuvant postdilation was more frequently performed in the OCT-guided arm. These findings suggest that OCT-guided PCI had a beneficial effect on stent strut coverage.
Although angiography guidance is widely performed during PCI, this method has only limited capability for accurately evaluating vessel size, lesion length, stent expansion, malapposition, and complications after stent implantation.15 The IVUS was introduced to overcome these limitations of angiography guidance, and may be useful for complex coronary interventions, such as those involving left main, bifurcation, and diffuse long lesions.16–19 The resolution of IVUS is 100μm in the axial plane, and 200-250μm in the lateral plane, whereas the resolution of OCT is ∼ 10-fold higher (10-15μm), which is sufficient to visualize individual stent struts and more detailed vascular structures. Consistent with this, neointimal coverage was visible on 99.9% of stent struts using OCT, vs only 25.8% using IVUS, since a considerable proportion of the neointima is < 100μm, which is thinner than the resolution of IVUS.20 Despite this, few prospective studies have assessed the potential benefits of OCT-guided PCI.
Based on analysis of autopsy samples, incomplete endothelialization and neointimal coverage over DES struts are proposed to be significant pathologic risk factors for stent thrombosis,1,2 and OCT and angioscopic studies identified a significant correlation between uncovered struts and subclinical intracoronary thrombus.21,22 Furthermore, the results of a recent retrospective case-control study using OCT suggest that the length of uncovered stent struts is a significant risk factor for late stent thrombosis.5 In addition, recent studies from our group tried to identify the cut-off value of uncovered struts that best predicted adverse clinical outcomes (cardiovascular death, myocardial infarction, and stent thrombosis) after DES implantation. During the median of 851 [interquartile range, 488-1215] days after OCT examination, clinical events occurred in 6 of 489 patients (4 definite stent thrombosis and 2 sudden cardiac death); the study proposed that the best cut-off value of uncovered struts percentage for predicting cardiovascular events was 5.9%, with a sensitivity of 83.3% and specificity of 70.3%.9 Consequently, identifying techniques to improve the extent of strut coverage may translate into a decreased incidence of adverse cardiovascular events and intracoronary thrombus during follow-up. Since acute stent malapposition is associated with delayed strut coverage of sirolimus-eluting stents at 10 months,6 minimizing acute stent malapposition may reduce the rate of uncovered stents later.
In the present study, we have tested this hypothesis using OCT guidance to optimize stent apposition and assessing stent strut coverage at 6 months. Our main finding was that the percentage of uncovered struts was significantly lower in the OCT-guided arm than in angiography-guided arm (1.60 [1.84]% vs 4.51 [5.43]%; P = .0004) at the 6-month follow-up. The proportion of uncovered struts and neointimal hyperplasia thickness following angiography guidance in the present study were comparable to our previous studies13,14 using zotarolimus-eluting stents (4.51% at 6 months [6.2% at 3 months and 3.3% at 9 months] and 96μm at 6 months [74μm at 3 months and 139μm at 9 months], respectively). These observations suggested that OCT guidance could decrease the incidence of uncovered struts with less variability at 6 months after stent implantation. In addition, the incidence of unfavorable strut coverage was significantly lower in the OCT-guided arm (2 patients [3.9%] vs 14 patients [25.9%]; P = .002) and the extent of stent strut coverage was less variable in OCT-guided arm. These findings suggest that the adoption of OCT-guidance during PCI will ensure consistently favorable strut coverage. The OCT-guided strategy may have a role to shorten the duration of dual antiplatelet therapy after DES-treated lesions with favorable strut coverage.23 Our findings are in accordance with a retrospective finding that OCT-guided PCI is associated with improved clinical outcomes at 12 months, due to reduced cardiac deaths and myocardial infarctions.24 Optical coherence tomography guidance is also suggested to provide benefits in other clinical settings when identifying neoatherosclerosis as the cause of stent restenosis or stent thrombosis.25,26 As is seen with IVUS guidance, OCT provides information during PCI on stent underexpansion and stent malapposition, which enabled adjuvant balloon dilation to be performed more frequently in the OCT-guided arm in this study (51.0% vs 27.8%; P = .03).
Study LimitationsOur study has some limitations. First, we cannot compare the incidence of acute stent malapposition associated with OCT and angiography guidance because postprocedure OCT examination was only performed in the OCT-guided arm. In addition, adjuvant postdilation for malapposition or stent expansion was not defined as a specific criterion but was performed at operator discretion in this trial. We need to investigate optimal OCT criteria to improve stent strut coverage, apposition, and clinical outcomes during follow-up. Second, our results are only informative for simple lesions, since patients with complex coronary lesions—diffuse long lesions, small (< 2.5mm) or large (> 4.0mm) vessel diseases, left main diseases, chronic total occlusion, and bifurcation lesions—were specifically excluded. Therefore, we cannot speculate that the impact of OCT guidance would be greater in complex lesions and cannot recommend the use of OCT in every patient with simple lesions because its cost-effectiveness is not fully identified yet. Currently this is actively being investigated, but other previous studies have already suggested a potential clinical benefit of OCT-guided PCI.24,27 Third, we only used zotarolimus-eluting stents and a follow-up of 6 months; therefore, any extrapolation of our findings to other DES and longer follow-up durations should be done cautiously. Finally, although the sample size was adequate to assess the stent strut coverage using OCT at 6 months follow-up, it was too small to evaluate the long-term clinical outcomes of OCT-guided PCI.
CONCLUSIONSUsing a randomized, prospective study, we have shown that OCT-guided PCI of a DES resulted in significantly improved strut coverage and decreased malapposed struts at 6-month follow-up compared to conventional angiography guidance.
FUNDINGThis study was supported by grants from the Korea Healthcare Technology R&D Project (Ministry for Health, Welfare and Family Affairs of the Republic of Korea) (A085012 and A102064), from the Korea Health 21 R&D Project (Ministry for Health, Welfare and Family Affairs of the Republic of Korea) (A085136), and the Cardiovascular Research Center from Seoul.
CONFLICTS OF INTERESTNone declared.