Excess weight is the most prevalent cardiovascular risk factor and certainly the factor that improves the least over time among those with established cardiovascular disease. The association between obesity and cardiovascular disease is complex and not limited to the standard risk factors like hypertension, dyslipidemia, and type 2 diabetes mellitus. In recent years, multiple studies have shown that obesity may cause cardiovascular diseases via multiple disease mechanisms like subclinical inflammation, endothelial dysfunction, increased sympathetic tone, atherogenic lipid profiles, enhanced thrombogenic factors and also through obstructive sleep apnea.
Despite the overwhelming data linking obesity to cardiovascular disease, several studies have shown a paradoxical association between obesity and prognosis among those with coronary disease and heart failure, which may be due to limitations of the way we currently define obesity. There is abundant data suggesting that measuring central obesity or total body fat content might be more appropriate than using the body mass index alone.
The management of obesity is challenging and studies using lifestyle modification alone or with pharmacologic agents generally have limited success and high levels of weight regain. Bariatric surgery has proven to be an effective and safe way to induce and maintain significant weight loss but is limited to those with medically complicated obesity or people who are severely obese.
Keywords
Obesity has become one of the major threats to health throughout the world. Its prevalence has increased in almost every continent and has probably increased in all the developed countries. Obesity, together with excess weight, is currently the most prevalent cardiovascular risk factor in individuals with established coronary heart disease.1 Obese individuals have poor quality of life and shorter life expectancy than normal-weight individuals. Epidemiological studies have shown that obesity is a major cardiovascular disease risk factor, including coronary heart disease, heart failure, atrial fibrillation, ventricular arrhythmias and sudden death. It is also considered a causal factor in hypertension, diabetes mellitus type 2, osteoarthritis, obstructive sleep apnea (OSA), dyslipidemia, gastroesophageal reflux, nonalcoholic fatty liver disease and many forms of cancer.2 The treatment of obesity places an immense economic burden on the health care system. The continuous increase in its prevalence has alerted public health officials, epidemiologists, and economists.3
In this article we discuss the fundamental aspects of the pathophysiology of obesity and its relation to cardiovascular disease, and summarize recent evidence that associates obesity with different forms of cardiovascular disease apart from coronary heart disease, such as atrial fibrillation, heart failure, and sudden death. We also review the current controversy on the way obesity is diagnosed. The diagnosis of obesity is usually based on estimates of the body mass index (BMI) and on the values used to define excess weight and obesity. The distribution of body fat has also been associated with cardiovascular events, and it may be more appropriate to measure total body fat and its distribution to determine the risk of obesity-associated cardiovascular disease.4 In this update, we summarize the best evidence related to the management of obesity, including the use of surgery.
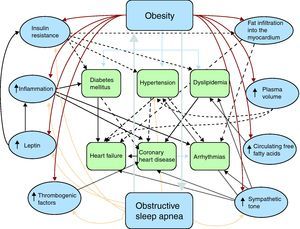
Pathophysiology of obesity and cardiovascular diseaseThe association between obesity and different forms of cardiovascular disease is complex, probably due to the different physiopathological mechanisms involving a large number of interacting factors (Figure 1). Obesity can cause coronary atherosclerosis through well-described and accepted mechanisms, such as dyslipidemia, hypertension, and diabetes mellitus type 2.5,6 However, recent evidence has shown that the association between obesity and cardiovascular disease7 could include many other factors, such as subclinical inflammation, neurohormonal activation with increased sympathetic tone,8 high leptin and insulin concentrations,9 OSA and increased free fatty acid turnover, and may also be due to fat deposits in specific areas of the body with a direct role in the pathogenesis of coronary atherosclerosis, such as subepicardial fat (Table 1).10
Figure 1. Pathophysiology of obesity and cardiovascular disease. The different physiopathological mechanisms by which obesity is associated with cardiovascular disease are complex and are not limited to factors such as diabetes mellitus type 2, hypertension or dyslipidemia. Other factors with an indirect interaction have been described, such as subclinical inflammation, neurohormonal activation with increased sympathetic tone, elevated leptin and insulin concentrations, obstructive sleep apnea, increased free fatty acid turnover and intramyocardial and subepicardial fat deposition.
Table 1. Metabolic and Cardiovascular Effects of Obesity.
| A. Increased insulin resistance |
| Glucose intolerance |
| Metabolic syndrome |
| Diabetes mellitus type 2 |
| Increased sympathetic tone |
| B. Hypertension |
| Increased plasma volume |
| C. Dyslipidemia |
| Elevated total cholesterol |
| Elevated triglycerides |
| Elevated LDLc |
| Elevated cholesterol other than HDLc |
| Elevated apolipoprotein B |
| Elevated small dense LDLc particles |
| Reduced HDLc |
| Reduced apolipoprotein A1 |
| Increased free fatty acid turnover |
| D. Abnormal left ventricular morphology |
| Concentric remodeling |
| Left ventricular hypertrophy |
| Fat infiltration into the myocardium |
| E. Endothelial dysfunction |
| F. Increase in systemic inflammation and prothrombotic state |
| G. Diastolic and systolic dysfunction |
| H. Heart failure |
| I. Coronary heart disease |
| J. Atrial fibrillation |
| K. Sudden death |
| L. Arrhythmias and ventricular ectopias |
| M. Obstructive sleep apnea and sleep-related breathing disorders |
HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol.
Excess visceral fat, associated with central obesity, is the metabolically most active adipose tissue that causes increased insulin resistance, high triglyceride concentrations, changes in the size of low-density lipoprotein (LDL) particles and low concentrations of high-density lipoproteins (HDL).11,12 The mechanisms by which excess fat causes insulin resistance are complex, certainly involving different physiopathological pathways, and are mediated by cytokines, other inflammatory mediators and elevated leptin levels. Insulin resistance causes diabetes mellitus type 2, a condition that alone can initiate or accelerate the atherogenic process by several additional mechanisms, such as hyperglycemia.13
Leptin is an important hormone in the induction of satiety. Resistance to leptin in obese humans is shown by increased serum leptin concentrations. It is a multiple-action hormone, whose possible effects include increased sympathetic activity that promotes thrombosis and increases blood pressure and heart rate. Leptin is a cytokine and is therefore also involved in the inflammatory process. Voluntary weight loss, especially the reduction of adipose tissue, leads to decreased circulating leptin.14
Activation of the Sympathetic SystemDirect measures of muscle sympathetic nerve activity and catecholamine concentrations suggest that obesity is associated with increased sympathetic activity.15,16 However, these studies are at variance with other clinical and experimental reports.17 Patients with morbid obesity, who usually have elevated sympathetic tone, commonly present OSA.18 Although OSA causes increased sympathetic activity, this disorder has not been taken into account in the majority of studies on sympathetic activity and obesity in humans, and thus it remains unknown whether the association between sympathetic activity and obesity is totally or partially mediated by sleep apnea. Increased sympathetic activity may be related to fat accumulating in the central region of the body, rather than to the BMI itself, or to a prolonged sedentary lifestyle or stress.19
Endothelial DysfunctionIncreased BMI and body fat content, especially central obesity, have been associated with endothelial dysfunction.20 The mechanisms by which obesity can induce endothelial dysfunction are not well defined. The endothelium is a complex organ with endocrine functions. It regulates smooth muscle cell proliferation, platelet function, vasomotor tone and thrombosis. Endothelial dysfunction induces chemotaxis of adhesion molecules and the differentiation of monocytes into macrophages. This is considered a key process in atherogenesis. Endothelial dysfunction also promotes platelet aggregation and decreases nitric oxide bioavailability, which promotes thrombosis by decreasing the ratio between plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator.21 Some experimental studies point out that sustained weight loss improves endothelial function.22,23
Systemic InflammationInflammation has emerged as a powerful predictor, and a possible etiological factor, of cardiovascular disease.24 Elevated C-reactive protein (CRP) has been associated with an increased risk of myocardial infarction, cerebrovascular disease, peripheral arterial disease and coronary heart disease death in apparently healthy men and women.25,26 It has also been suggested that obesity is an inflammatory state. A positive association has been observed between BMI and CRP in adults and children.27 The mechanisms by which obesity leads to elevated CRP have not been fully explained. Interleukin-6 (IL-6) is a cytokine that stimulates the production of CRP in the liver. IL-6 is produced and released by adipose tissue into the bloodstream and a strong correlation has been demonstrated between serum CRP concentrations and IL-6 content in adipose tissue in humans.28 Of note, the release of proinflammatory cytokines (such as IL-6) by adipose tissue may be influenced by leptin. Experimental studies in rats suggest that CRP can induce atherosclerosis and is not only an indirect marker of vascular inflammation.29,30
Changes in Hemostatic FactorsObesity is associated with various changes in the coagulation and fibrinolytic systems. Obese individuals have increased concentrations of fibrinogen, factor VII, factor VIII, von Willebrand factor and PAI-1 and increased platelet adhesion than lean individuals.31,32,33 It has been postulated that obesity induces changes in hemostasis and fibrinolysis by various mechanisms, such as an increased inflammatory state with the subsequent increase of fibrinogen, insulin resistance and endothelial dysfunction (von Willebrand factor and factor VIII), and by regulating PAI-1 production by adipose tissue or by the effect of several cytokines, hormones, and growth factors.34,35 Furthermore, some epidemiological studies have suggested that the association between obesity and changes in coagulation factors could be limited to the central distribution of fat and not to the BMI, with possible interactions with insulin resistance.36,37 Data are scarce and are more controversial in studies that associate weight loss with changes in coagulation factors.
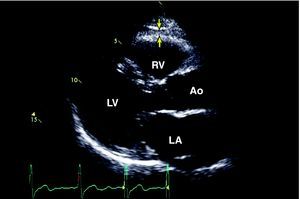
Paracrine Effect of Subepicardial FatSubepicardial adipose tissue is a particular type of visceral adipose tissue deposited around the heart, particularly around the subepicardial coronary arteries (Figure 2). Although at first glance this description may seem to be of anatomical interest alone, evidence has suggested the physiological and metabolic importance of this type of adipose tissue, especially its association with cardiovascular risk and the pathogenesis of coronary atherosclerosis.10,38 Studies in cadavers have demonstrated that the weight of subepicardial fat is associated with total heart weight, and that coronary atherosclerotic plaque tends to be more prominent on the side of the arteries in contact with the fat deposits. Other studies have shown that subepicardial fat supplies free fatty acids for energy production and cytokine synthesis. Animal studies suggest that the rate of fatty acid synthesis is greater in subepicardial fat than in other body sites.39,40 Furthermore, it has been shown that the subepicardial fat of patients with severe coronary heart disease is a source of various inflammatory mediators and has a marked response to inflammation, independent of BMI or diabetes.41 Our group has already shown that the interatrial septum thickness, which is related to the quantity of subepicardial fat, correlates with the angiographic presence and severity of coronary heart disease.42 Subepicardial fat, measured on the right ventricle or around the heart, is also related to waist circumference, diastolic blood pressure, left ventricular mass, elevated insulin concentrations, glucose uptake and the severity of the coronary heart disease as assessed by coronary angiography.43,44,45,46,47
Figure 2. Outline of subepicardial fat by two-dimensional echocardiography in the parasternal long-axis view. 38 The arrows identify the echoic space between the subepicardium and pericardium. Ao, aorta; LA, left atrium; LV, left ventricle RV, right ventricle.
De Vos et al48 examined the specific location of subepicardial fat in post-menopausal women using CT scan, and demonstrated an association between the subepicardial fat directly surrounding the coronary arteries and various vascular risk factors, as well as the lack of an association between subepicardial fat and BMI. The authors also found that pericoronary fat is associated with coronary calcification.
It is highly likely that inflammatory mediators outside the coronary artery, such as pericardial and adventitial inflammation, may contribute to atherosclerotic lesions. The close relationship between subepicardial fat and the adjacent coronary arteries enables paracrine interaction between these structures.38
Even though various studies have suggested that subepicardial fat may play a role in the development of coronary heart disease, the exact mechanism requires further study.
Obstructive Sleep Apnea as a Mediator between Obesity and Cardiovascular DiseaseOSA is characterized by repeated episodes of cessation of breathing followed by sudden awakening.49 OSA has been specifically associated with hypertension, ischemic heart disease, cerebrovascular disease, heart failure, pulmonary hypertension and cardiac arrhythmias.50 Patients with OSA usually present associated factors, such as obesity, hypertension, and insulin intolerance. Longitudinal studies that have evaluated the incidence of cardiovascular disease and studies that have assessed the effects of continuous positive airway pressure therapy have suggested a causal association between OSA and several cardiovascular disorders. OSA causes acute and chronic stress that could predispose to myocardial ischemia during sleep. Acute hypoxia increases the peripheral vasoconstriction induced by apnea and severe hypoxemia; carbon dioxide retention, sympathetic activation and the sudden increases in blood pressure could equally cause myocardial ischemia. In patients with OSA, the peak incidence of myocardial infarction occurs during the sleep period, whereas its incidence is at its lowest during this period in the general population.51 In the long term, the development of hypertension, which initially manifests at night and subsequently during the day, the production of vasoactive and trophic substances such as endothelin, the activation of inflammatory and procoagulant mechanisms and the increase of insulin concentrations can also contribute to the development and progression of ischemic heart disease. In the Sleep Heart Health Study cohort, OSA was found to be an independent risk factor of coronary heart disease; these findings were confirmed by additional prospective studies.52 Other studies restricted to patients with coronary heart disease have demonstrated an association between OSA or snoring and the subsequent risk of infarction, and thus OSA may be a prognostic indicator in these patients.53
Cardiovascular Complications Of Obesity Obesity and Coronary Heart DiseaseObesity, and excess weight, is the most common cardiovascular risk factor in patients who have suffered a myocardial infarction. More than two-thirds of the patients with coronary heart disease are overweight or obese.7,54 The progress made during the last 30 years in controlling various cardiovascular risk factors, such as smoking and dyslipidemia in patients with coronary heart disease, has not been matched by progress in managing excess weight. Overweight individuals are rarely diagnosed with obesity by their physicians.55 This is also certainly the case for individuals with a history of cardiovascular disease.56,57
Compared to individuals with normal weight, obese patients with coronary heart disease are at increased risk of dyslipidemia and hypertension, more often have a sedentary lifestyle and are usually 10 years younger; thus, they provide a unique opportunity to implement secondary prevention interventions.58
The association between obesity and coronary heart disease is partially mediated by traditional risk factors such as hypertension, dyslipidemia and diabetes mellitus, although these risk factors do not fully explain this association. Coronary atherosclerosis probably begins or is accelerated by various mechanisms potentiated by obesity, such as increased sympathetic tone, increased circulating free fatty acids, increased intravascular volume with higher vascular wall stress, inflammation, and changes in lipoproteins that increase their atherogenic potential. As mentioned, OSA may mediate this association. The prothrombotic state in obese individuals probably contributes to the onset of acute coronary events.59 Insulin resistance may be another mediator of obesity and cardiovascular disease, particularly in individuals with metabolic syndrome.13
Obesity, Heart Failure and CardiomyopathyEvery year in the USA, more than 400,000 new cases of heart failure are diagnosed and approximately 3 million individuals present symptoms of heart failure, making it the new cardiovascular epidemic of the 21st century. It has been proposed that the prevalence of obesity could be partly due to the increased incidence of heart failure in recent years, not only because of the parallel increase in both diseases, but based on the epidemiological and mechanical evidence that links them.60 Obese individuals have double the risk of heart failure compared to individuals with a normal BMI.61 Patients with advanced obesity who suffer heart failure without an identifiable cause of left ventricular dysfunction are diagnosed as having obesity-associated cardiomyopathy.62
For several years it was believed that obesity could cause heart failure only via intermediary mechanisms such as hypertension or coronary heart disease, but recent studies have demonstrated that other factors could be implicated in the origin of obesity-associated cardiomyopathy. For example, there is obesity-associated left ventricular hypertrophy, which cannot be explained by the increase in blood pressure alone. Animal and human studies have demonstrated an increase in the prevalence of myocardial fibrosis that is proportional to the degree of obesity and is associated with cellular degeneration and inflammation.63 Furthermore, obesity has also been associated with diastolic dysfunction, which represents 50% of the cases of heart failure. Recent studies have also demonstrated that patients with central obesity can develop fat infiltration into the myocardium that can evolve into fibrosis and diastolic or systolic left ventricular dysfunction.64
Obesity and Atrial FibrillationThe prevalence of atrial fibrillation and obesity has significantly increased in recent years. The increased prevalence of atrial fibrillation could be due to population aging in combination with a better prognosis of patients with hypertension, coronary heart disease and heart failure, disorders that increase the risk of atrial fibrillation.65 Various studies suggest that obesity can cause or favor the appearance of atrial fibrillation. A recent metaanalysis66 of 16 studies with 123,000 patients assessed the impact of obesity on atrial fibrillation and demonstrated that obese patients have a 50% higher risk of atrial fibrillation and that risk increases as BMI increases. On the other hand, studies on postoperative cardiac patients have not demonstrated any increase in the risk of atrial fibrillation in obese patients.
Obesity and Ventricular ArrhythmiasSome clinical trials suggest that obesity is associated with sudden death. Although progression to heart failure may be the most common cause of death in patients who have obesity-associated cardiomyopathy, it has also been reported that sudden death is more common in apparently healthy obese patients than in lean individuals.5 Electrophysiological studies in obese individuals have demonstrated increased electrical irritability that can trigger the onset of ventricular arrhythmias, even in the absence of ventricular dysfunction or clinical heart failure.67 In the Framingham study, the annual rate of sudden cardiac death in obese patients was nearly 40 times greater than in the non-obese population.67
Other physiopathological mechanisms could also be implicated in the association between obesity and sudden death or ventricular arrhythmias. There is a direct correlation between corrected QT interval and BMI. Similarly, the prevalence and number of abnormal late evoked potentials, factors which are associated with a high risk of sudden death, are increased in individuals with morbid obesity.68 The presence of late potentials in obese patients could be secondary to the well-known changes in obesity-associated cardiomyopathy, such as fibrosis, fat and mononuclear cell infiltration, and myocyte hypertrophy.69 In general, obese individuals have a faster heart rate and reduced heart rate variability due to abnormalities in sympathovagal balance, factors which are associated with an increased risk of sudden death.70
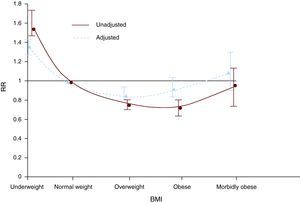
The obesity paradoxThere is a direct and J-shaped relationship between BMI and the incidence of coronary heart disease. However, once coronary heart disease is manifested, the association between BMI and prognosis becomes more complex. This has been called the obesity paradox since, contrary to expectation, several studies have demonstrated that individuals with established coronary heart disease and excess weight or mild obesity have a better prognosis than low- or normal-weight individuals. These findings, originally described in isolated reports, were subsequently confirmed in a metaanalysis that assessed more than 250,000 patients with coronary heart disease (Figure 3).7 These findings should be examined with caution before concluding that excess body fat is not a risk factor for coronary heart disease progression or that it is a protective factor. First, observational studies that demonstrate the paradox do not prove that weight loss is not of benefit to obese patients with coronary heart disease, since all the studies used only baseline bodyweight values in the analysis, without follow-up data. Other paradoxical associations exist among individuals with coronary heart disease, such as the case of smokers.71 It has been demonstrated that smokers have a better prognosis following myocardial infarction than individuals who have never smoked.72 However, it has also been shown that smokers have low levels of coronary heart disease because they usually have single-vessel disease.73 Furthermore, smokers who quit the habit have a much better prognosis than current smokers.74 Thus, considering the obesity paradox from the perspective of the smoker's paradox, it can be argued that weight loss could be of benefit to obese individuals with coronary heart disease. This was confirmed in an observational study of patients who had undergone cardiac rehabilitation, because those who lost weight, independent of their baseline weight, had a better prognosis than patients whose weight increased or did not change.75
Figure 3. Adjusted and unadjusted relative risk (RR) of overall mortality in individuals with coronary heart disease by body mass index (BMI) groups. 7
The obesity paradox has been accounted for in different ways. Low BMI values are associated with low lean muscle mass, also known as sarcopenia.76 Patients with sarcopenia have reduced exercise capacity, reduced VO2 max and other characteristics commonly associated with greater mortality. Since BMI cannot differentiate between muscle mass and fat, individuals who have coronary heart disease and are moderately overweight or obese may have more preserved muscle mass. When BMI reaches very high values, which provide a better indication of body adiposity,77,78,68 the obesity paradox disappears. Our group recently conducted a metaanalysis that used individual patient information from 5 cohorts, and demonstrated that although central obesity in individuals with coronary heart disease does not have a paradoxical association, there is a direct association between central obesity and mortality (unpublished data). These findings also support the idea that measures of central obesity are better markers of fat-related cardiovascular risk, particularly in patients with coronary heart disease.
Diagnosis of ObesityFor many years, ideal weight and the percentage of excess weight was determined according to the actuarial tables of the Metropolitan Life insurance company.79 It is only during the last 20 years that BMI has become the most widely applied tool to diagnose obesity. Defined as weight in kilograms divided by the square of height in meters, BMI was first described by a Belgian mathematician in the 19th century . 80
In 1995, the World Health Organization (WHO) defined obesity as a BMI ≥ 30, which was based on a consensus of scientists and experts. This cutoff was selected because the mortality curve in several epidemiological studies increased at this value. The WHO81 also defined overweight as a BMI ≥25.
Limitations of the Tools Used to Diagnose ObesitySeveral studies have compared the use of the BMI to determine body fat with techniques known to accurately measure body composition. The results of these studies have varied, but the majority demonstrated that the standard BMI cutoff values used to define obesity appear to underestimate body fat. A BMI ≥ 30 has a sensitivity of 50% to detect excess fat, and thus half of the individuals with a high percentage of body fat will not be considered obese. Furthermore, since total weight is used in the denominator to calculate BMI, some individuals with preserved muscle mass and little fat are diagnosed as being overweight. On the other hand, the BMI does not take body fat distribution into account, and thus some individuals who are normal weight or slightly overweight but who have abnormal body-fat distribution, and who could be at an increased risk of cardiovascular events, diabetes mellitus type 2 and overall mortality, would not be considered at risk according to these criteria.82
Measures of Central ObesityThe waist-to-hip ratio has been used as a proxy measure of body fat distribution to assess health issues associated with obesity. Measures of central obesity are very useful in improving the assessment of obesity-associated risk. Central obesity usually refers to increased abdominal fat. However, some researchers have suggested that the term also refers to the distribution of truncal or axial fat, which includes visceral adiposity and subcutaneous fat from the abdomen, thorax and proximal segments of the upper limbs. Central obesity is associated with excess visceral fat. This appears to be the metabolically most active fat and is a cause of insulin resistance, hypertriglyceridemia, small LDL particles and low HDL concentrations, all of which are considered to be proatherogenic factors.12
Different methods for measuring abdominal circumference have been proposed. Some include the perimeter of the abdominal wall above the upper edge of the iliac crest, others use the umbilical scar as a reference point, whereas some researchers have used the greatest abdominal circumference regardless of its location. All these methods correlate well with the total quantity of visceral fat in grams as measured by more accurate techniques such as computed tomography or magnetic resonance imaging. Hip circumference is measured at the level of the major trochanters or at the greatest circumference at the level of the buttocks. The standard cutoffs used to define central obesity are listed in Table 2.
Table 2. Diagnostic Criteria for Obesity and Central Obesity According to Different Methods.
| Category | Body mass index [c.b.] |
| Underweight | ≤18.5 |
| Normal weight | 18.5-24.9 |
| Overweight | 25-29.9 |
| Obesity class I | 30-34.9 |
| Obesity class II | ≥35 |
| Central obesity by abdominal circumference * | |
| Population | Cutoff |
| Euroamerican men | ≥102cm (40″) |
| Euroamerican women | ≥88cm (35″) |
| Asian men | ≥90cm (35″) |
| Asian women | ≥80cm (32″) |
| Central obesity by waist-to-hip ratio | |
| Men | >0.9 |
| Women | >0.85 |
According to the American Heart Association/National Heart, Lung and Blood Institute (Adult Treatment Panel III), these ranges are also recommended for white individuals; there is no evidence supporting the use of different values for Hispanic Americans, black Americans or American Indians.
* Recommended cutoffs for other groups: for the Japanese population, the Japanese Obesity Society suggests ≥85cm for men and ≥90cm for women; the Cooperative Task Force suggests ≥85cm for Chinese men and ≥80cm for Chinese women; the International Diabetes Association suggests≥94cm for men and ≥80cm for women from Middle Eastern, Mediterranean and Sub-Saharan populations, and ≥90cm for men and ≥80cm for ethnic Central and South American populations.
The diagnosis of central obesity has several limitations. It remains unclear whether the waist-to-hip ratio provides better prognostic information than abdominal circumference alone, and there is a great deal of controversy over which of the 2 measurements has a stronger association with mortality, diabetes mellitus or cardiovascular disease. Abdominal circumference has shown reasonable reproducibility in research studies, but variability could be a significant issue in clinical practice. The fact that there are various ways of measuring abdominal circumference is also a source of inconsistency.12
Body Fat ContentAlthough the word obesity is defined as excessive fat, in clinical practice obesity is not diagnosed by measuring fat or body composition. In fact, there is no consensus on the percentage of body fact considered to be normal. Researchers in this field generally define excess fat as >30%-35% in women and >20%-25% in men.
The methods used to calculate body fat composition—specifically body fat percentage and lean mass content—have traditionally been considered very complex (eg, water-immersion plethysmography, isotope dilution techniques) or inaccurate (eg, skinfold method, body impedance analysis using commercial equipment). However, other methods, such as dual energy X-ray absorptiometry, multi-frequency bioimpedance analysis and air-displacement plethymsography are relatively simple, reproducible and valid.83 Although there is data on the use of these methods in clinical practice, it would appear that only a minority of medical institutions use them to assess body adiposity.
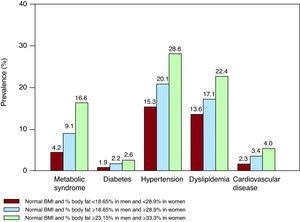
Normal Weight ObesitySome recent reports suggest that individuals with a normal bodyweight, as defined by BMI, could be at risk of metabolic syndrome, cardiometabolic dysregulation and even greater mortality. A recent study demonstrated that normal-weight men in the upper tertile of body fat percentage (>23% body fat) have a 4-fold increased risk of metabolic syndrome and have a greater prevalence of diabetes mellitus, hypertension, dyslipidemia and cardiovascular disease than those in the lower tertile. Women in the upper tertile of body fat (>33% body fat) have a 7-fold increased risk of metabolic syndrome (Figure 4). Curiously, normal-weight obese women are almost twice as likely to die during follow-up than women in the lower percentile of body fat.84 Although further studies are needed to confirm these results, it is clear that individuals with normal weight based on BMI may require a more detailed classification method to better define their cardiometabolic risk related to adiposity.
Figure 4. Prevalence of metabolic syndrome, risk factors and history of cardiovascular disease in normal-weight individuals by percentile body fat. Individuals in the highest tertile (green) would be classified as normal-weight obese. 77 BMI, body mass index.
Obesity therapy Medical TherapyThe main aim of obesity therapy is weight loss and maintenance by dietary interventions and increased physical activity. However, maintaining the weight loss using these interventions is difficult and relapse rates are high. In recent years, the use of adjuvant drugs and lifestyle modification in patients with a BMI ≥30 have been proposed, especially if they already have obesity-associated diseases.85 A metaanalysis conducted by Rucker et al86 assessed the efficacy and adverse effects of long-term therapy using orlistat, sibutramine and rimonabant. Orlistat, a gastrointestinal lipase inhibitor, reduced weight by 2.9% compared to placebo.
On the other hand, sibutramine, a centrally acting monamine reuptake inhibitor, reduced weight by 4.3% compared to placebo, and 10%-30% of the patients successfully maintained the weight loss. This differed from the results of studies that assessed orlistatin, in which all patients, whether they received orlistat or placebo, showed similar amounts of weight regain. Patients receiving rimonabant, a CB1 endocannabinoid receptor antagonist, lost 4.7kg more weight than those taking placebo, and maintained the weight loss.
Apart from weight loss, these drugs also reduce abdominal circumference and systolic and diastolic blood pressure. Orlistat specifically reduced the incidence of diabetes mellitus, fasting glucose and glycosylated hemoglobin (HbA1c) concentrations in diabetics, total cholesterol, LDL cholesterol and HDL cholesterol (HDLc). Sibutramine and rimonabant reduced triglyceride concentrations and increased HDLc concentrations.
When medical therapy and lifestyle modification fail or the individuals are morbidly obese, other measures to induce weight loss are recommended, such as bariatric surgery.
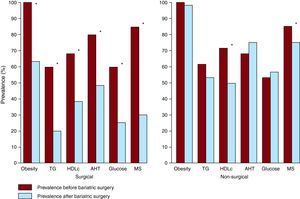
Bariatric SurgeryIt has been demonstrated that bariatric surgery is an effective and safe technique for weight loss in patients with morbid obesity or obesity and comorbidities, such as coronary heart disease.87 Currently, there are 2 main techniques: vertical banded gastroplasty and Roux-en-Y gastric bypass. More than half of the patients who undergo bariatric surgery, especially those referred to gastric bypass, lose at least 50% of the excess weight.88 Some studies suggest that bariatric surgery can also lead to significant improvements in blood pressure, glycemia, lipid concentrations and quality of life (Figure 5). More than 70% of the hypertensive patients presented improved or controlled blood pressure values.89 About half of the diabetic patients who undergo bariatric surgery can achieve normal HbA1c levels and can even stop using insulin or hypoglycemic agents.90 Patients undergoing bariatric surgery have significantly reduced estimated cardiovascular risk and some studies suggest that there may be reductions in mortality following bariatric surgery.91
Figure 5. Changes in the prevalence of metabolic syndrome and in each of the 5 components that define it, according to the American Heart Association/National Heart, Lung and Blood Institute (AHA/NHLBI), after bariatric surgery or after non-surgical interventions. 90 AHT, arterial hypertension; HDLc, high-density lipoprotein cholesterol; MS, metabolic syndrome; TG, triglycerides.*Statistically significant changes (P<.05).
Changes in Cardiac and Mechanical Function After Bariatric SurgeryAlthough several studies have demonstrated abnormalities in cardiac structure and function in obese patients, few studies have assessed the effect of bariatric surgery on cardiac morphology. It has been shown that left ventricular mass decreases and right ventricular function improves after weight loss following bariatric surgery.92 It has also been shown that bariatric surgery can arrest deterioration in left ventricular diastolic function, as assessed by left atrial size.93 However, some studies that have assessed changes in left ventricular ejection fraction (the most accepted measure of cardiac function) have not demonstrated significant improvements following bariatric surgery. It has been demonstrated that strain and strain rate are very abnormal in patients with class II or III obesity compared to normal-weight individuals, and that this abnormality could resolve after bariatric surgery.94 It remains to be demonstrated whether these changes in cardiac function are due to the improvement in hypertension, OSA and diabetes or the reduction of body fat.
ConclusionsObesity is a common cardiovascular risk factor which is frequently ignored by physicians. Obesity is associated with several cardiovascular diseases and is not only linked to coronary heart disease, but also to abnormalities in heart rate and ventricular function. This association is due to multiple mechanisms and not only to hypertension, diabetes mellitus or dyslipidemia. The diagnosis of obesity should include measurements of total body fat content and its distribution. Although the management of obesity is difficult, a comprehensive management program can lead to favorable outcomes.
Conflicts of interestNone declared.
Corresponding author: Division of Cardiovascular Diseases, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 USA. lopez@mayo.edu