The term nonvalvular atrial fibrillation (NVAF) is used with increasing frequency to describe patients who may benefit from new oral anticoagulants (NOACs). This is a cause for concern for us because the European guidelines for the management of atrial fibrillation, dated 2012,1 state that “no uniform or satisfactory definition of these terms exists.” The indication for NOACs is based on 4 pivotal studies. To clarify this concept, we have reviewed the inclusion criteria in the protocols of these studies in terms of native valve lesions:
- •
The RE-LY study2 did not include the term NVAF. Patients with “hemodynamically relevant valve disease” were excluded and, as far as we are aware, a more precise definition was not included.
- •
The ROCKET trial3 was the only study that included the term NVAF. However, the protocol only excluded patients with “hemodynamically significant” mitral valve stenosis. For the indication of rivaroxaban, atrial fibrillation with a valve lesion other than mitral valve stenosis would not be considered NVAF.
- •
The authors of the ARISTOTLE trial4 and ENGAGE AF-TIMI 48 trial5 did not use the term. Both these trials excluded only patients with moderate or severe mitral valve stenosis.
A patient with severe aortic stenosis or mitral valve regurgitation with atrial fibrillation would not have been excluded from 3 of the 4 pivotal trials due to valve lesions. It would appear striking and inconsistent to describe such a patient as having NVAF. With valve disease, generalizations are inappropriate. Thus, thromboembolism as a pathophysiologic mechanism for mitral stenosis cannot be considered similar to mitral valve regurgitation or pulmonary stenosis.
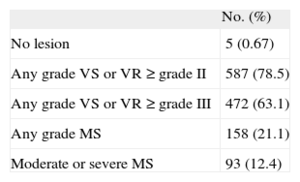
The use of a poorly defined term may lead to problems for certain therapeutic indications. To quantify the problem, we reviewed the echocardiography database of a secondary university hospital with no heart surgery facilities. In the last 6 months of 2013, echocardiograms were recorded for 748 patients with atrial fibrillation but without a valve prosthesis, and with a CHADS2 score of 1 or more. The patients with valve lesions are shown in the Table.
Valve Lesions in 748 Patients With Atrial Fibrillation but Without Prostheses
| No. (%) | |
| No lesion | 5 (0.67) |
| Any grade VS or VR ≥ grade II | 587 (78.5) |
| Any grade VS or VR ≥ grade III | 472 (63.1) |
| Any grade MS | 158 (21.1) |
| Moderate or severe MS | 93 (12.4) |
MS, mitral valve stenosis; VR, regurgitation of any valve; VS, stenosis of any valve.
In terms of their valve lesions, 655 patients (87.6%) would correspond to the clinical profile of ARISTOTLE4 and ENGAGE AF-TIMI 48 trials.5 Between 590 and 655 (78.9% and 87.6%) would correspond to the ROCKET profile.3 It is more difficult to determine how many would correspond to the RE-LY profile2 and how many would have NVAF. This would depend on the threshold for NVAF. If NVAF requires “hemodynamically relevant valve disease”, between 161 and 276 patients could be included (21.5%-36.9%). These notable differences highlight the weakness of the term NVAF when selecting patients for treatment with NOACs.
These data cannot be extrapolated to the general population because the patients were referred for echocardiography. Nevertheless, they may be representative of a large proportion of patients with different types of valve lesions and atrial fibrillation.
The authors of the ARISTOTLE trial reported that more than a quarter of the patients in the study had valve lesions that could be considered significant.6 In these patients, the benefit of apixaban was similar to that in patients without valve lesions. This finding may dissipate doubts about the risk of using NOACs, or at least apixaban, in patients with valve lesions other than mitral stenosis. In the ROCKET trial, 14% of the patients were considered to have significant valve lesions.
The clinical trials show that NOACs, or at least factor Xa antagonists, can be used in patients with atrial fibrillation who do not have mechanical prostheses2–5 or significant mitral valve stenosis,3–6 although they may have other valve lesions, whether or not they are significant. This point is essential, as atrial fibrillation is the most frequent sustained arrhythmia, while mitral valve stenosis is becoming less frequent. The term NVAF does not seem appropriate as an umbrella term for patients who may benefit from NOACs. Not only is this term not representative, but it is also not defined in the guidelines and may lead to inappropriate and uneven clinical management in the indications for anticoagulation. We are therefore of the opinion that this poorly defined term should not be used in this context.
CONFLICTS OF INTERESTA. García Lledó has provided scientific advice to Bristol-Myers and Bayer and has given presentations and classes in courses sponsored by Pfizer and Boehringer.