Keywords
INTRODUCTION
Traditionally, persons with type-1 diabetes mellitus (DM1) usually have a normal or low weight and a tendency for the late development of arterial hypertension and dyslipidemia. Until a few years ago, the main causes of death in persons with DM1 were those derived from microvascular complications, particularly kidney disease.1 Although therapeutic advances for the treatment of DM1 and its main associated cardiovascular risk factors have led to significant reductions in the rates of death and microvascular complications,2-5 no similar trend has been seen with cardiovascular disease.6
Though DM1 involves the immune destruction of the pancreatic beta cells and usually presents at an early age, affected persons are not exempt from developing insulin resistance at some earlier stage in the course of the disease,7-9 which is known as "double diabetes." This phenomenon usually appears in patients with a family history of type 2 diabetes mellitus (DM2) and is associated with a greater body mass index, greater insulin requirements and worse metabolic control.10 As well as the insulin resistance, other factors can explain why cardiovascular disease is the leading cause of death in these patients, such as the chronic complications of DM1, like kidney disease,11 and the earlier age of onset of the disease, with the resulting longer period of exposure to the main cardiovascular risk factors, together with a poor control of these risk factors.12,13 As a consequence, the phenotypic characteristics and cardiovascular risk profile of patients with DM1 have become more similar to those of DM2 over recent years.
Since the first description of the metabolic syndrome, by Reaven in 1988,14 numerous studies have established its association with a high prevalence of cardiovascular disease and death.15-18 About 10.2% of the Spanish working population have the metabolic syndrome, and this figure rises to 58.4% in persons with DM2 and 50.4% in persons with impaired fasting glucose.19 Most studies have been undertaken in patients aged 40 years or over, many with DM2 or impaired fasting glucose, with very few data available for patients with DM1. Given the lack of relevant studies in our setting, we examined the prevalence of the metabolic syndrome in patients with DM1 and its possible related factors.
METHODS
Patients
We undertook a cross-sectional study of patients with DM1 seen consecutively between January and December 2008 at the Outpatient Endocrinology Clinic of the Hospital del Mar in Barcelona, Spain. DM1 was considered to be autoimmune when it fulfilled the diagnostic criteria for diabetes mellitus20 together with positive tests for anti-GAD/65 Ks or anti-IA2 antibodies at the start and a concentration of free C-peptide <1.1 ng/mL 6 min after intravenous administration of 1 mg of glucagon. Non-Caucasian patients were excluded, as were patients with variations in the concentration of glycosylated hemoglobin >1% at 3 bimonthly determinations, pregnant women, patients who had an excessive consumption of alcohol, patients with chronic end-stage kidney failure, kidney transplant recipients or those on hemodialysis. No patient was being treated with insulin sensitizing drugs, such as thiazolidinediones or metformin. The study protocol, approved by the hospital Ethics Committee, included a physical examination and a blood test. All the participants were aged 18 years or over and had had diabetes for longer than 6 months.
Data were recorded for each patient on age, sex, time since the diagnosis of diabetes, history of major cardiovascular events (acute myocardial infarction, coronary revascularization procedures, angioplasty, stroke, transient ischemic attack, and peripheral vascular disease, defined as the presence of intermittent claudication or amputation), as well as the presence of chronic microangiopathic complications of the diabetes (microalbuminuria or macroalbuminuria, retinopathy, peripheral or autonomic neuropathy). The presence of complications was evaluated by an expert diabetologist (JJC), except for retinopathy, which was assessed by an ophthalmologist. The criteria of the American Diabetes Association were used for the clinical diagnosis of complications,20 and the insulin requirements were estimated in units per kilogram of body weight (U/kg/d). The physical examination included measurements of weight, height and abdominal waist circumference, as well as the blood pressure using standardized methods.
The renal status was classified from the urinary albumin excretion (UAE): a) absence of kidney disease was defined as normoalbuminuria (UAE<30 mg/24 h); b) incipient kidney disease as microalbuminuria (UAE 30-300 mg/24 h); and c) established kidney disease as macroalbuminuria (UAE>300 mg/24 h). The UAE was expressed as the mean of three 24 h urine samples taken at the patient's home during a period of normal activity on 2 separate occasions, at least 1 month apart.
Measurements
All the patients included in the study had venous blood drawn after a 12 h fast. Measurements were made of the concentrations of total cholesterol and triglycerides using enzymatic methods in a Cobas Mira automatic analyzer (Baxter Diagnostics AG, Düdingen, Switzerland) and separation of high density lipoprotein cholesterol (HDL-C) by phosphotungstic acid and magnesium chloride precipitation. Blood glucose was measured by the glucose oxidase method. Glycosylated hemoglobin was quantified by chromatography (Biosystem, Barcelona, Spain) and the urinary excretion of albumin by nefelometry (intra-assay coefficient of variation, 2%). Free C-peptide was determined by radioimmunoassay 6 min after the intravenous administration of 1 mg of glucagon, in a fasting state and with a baseline glycemia <180 mg/dL to avoid beta cell glucotoxicity, at least 1 month after starting insulin therapy. The prior insulin dose was administered the night before.
Criteria for the Metabolic Syndrome
In accordance with the modified criteria of the National Cholesterol Education Program -Adult Treatment Panel III (NCEP-ATP III),21 the metabolic syndrome was diagnosed if the patient had 3 or more of the following conditions: fasting plasma glucose ≥100 mg/dL or treatment with glucose lowering dugs, arterial blood pressure ≥130/85 mmHg or antihypertensive medication, fasting plasma triglycerides ≥150 mg/dL (1.7 mmol/L) or drug treatment for hypertriglyceridemia, HDL-C <40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women or drug therapy to raise the HDL-C concentration, and an abdominal waist circumference ≥102 cm in men and ≥88 cm in women.
Statistical Analysis
For an alpha risk of .05 and precision of ±10% in a bilateral contrast for an estimated 40% rate of the metabolic syndrome and assuming a population of 100 000, we required a random sample of 93 persons.
The Student t test was used to compare means and the χ2 test for categorical variables, as well as the Mann-Whitney U test for variables that did not follow a normal distribution, and Pearson's correlation coefficient to establish relations between quantitative variables. To evaluate the factors associated with the presence of the metabolic syndrome (dependent variable), a multiple logistic regression model was applied that included as independent variables those which had a P<.1 in the univariate analysis. The results were analyzed using the statistical program SPSS, version 12.0 for Windows.
RESULTS
Of the 165 patients seen at the Outpatient Endocrinology Clinic during 2008, 56 were excluded due to lack of confirmation of the diagnosis of autoimmune DM1, variations in the glycosylated hemoglobin concentration, excessive consumption of alcohol or end-stage renal failure. Of the 109 patients eligible, 91 (83.5%) completed the study protocol and composed the definitive sample. The age (mean [standard deviation]) of the patients was 39.7 (13.2) years; 53 were men and 38 women, with a mean duration of DM1 of 16.7 (12.9) years and a mean glycosylated hemoglobin concentration of 7.29% (1.4%).
All the patients fulfilled the criterion of high fasting plasma glucose; 57 (62.6%) fulfilled 2 or more criteria; 29 (31.9%), 3 or more; 11 (12.1%), 4 or more; and 2 (2.2%) fulfilled all the criteria for the metabolic syndrome. Thus, 29 patients (17 men, 12 women) had the metabolic syndrome according to the NCEP-ATP III modified criteria,21 giving an overall prevalence of 31.9% (95% confidence interval [CI], 22.3-41.5). Table 1 shows the prevalence of each of the components of the metabolic syndrome in the whole group of patients with DM1.
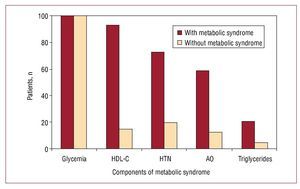
The most frequent criterion among the patients with the metabolic syndrome, besides glycemia, was the HDL-C concentration, present in 93.1% of the cases, followed by hypertension (72.4%), abdominal obesity (58.6%) and hypertriglyceridemia (20.7%). These percentages contrast with those seen in the patients with DM1 but without the metabolic syndrome (Figure 1).
Figure 1. Proportion of patients who fulfilled the different criteria for the metabolic syndrome. HDL-C indicates high density lipoprotein cholesterol; HTN, arterial hypertension; AO, abdominal obesity.
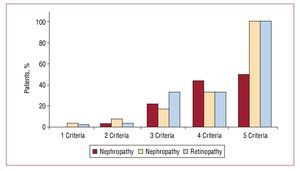
The patients with DM1 and the metabolic syndrome were older and had longer duration of diabetes, higher body mass index and a greater prevalence of overweight than those DM1 patients without the metabolic syndrome (Table 2). No differences were found in the percentage of macroangiopathic complications. However, the patients with DM1 and the metabolic syndrome had a significantly higher prevalence of microangiopathic complications (retinopathy, neuropathy and nephropathy) than the DM1 patients without the metabolic syndrome. In addition, a direct relation was detected between the number of components of the metabolic syndrome and the prevalence of microangiopathy, reaching 100% in those patients who had all the diagnostic criteria for the metabolic syndrome (Figure 2). On the other hand, the daily insulin requirements were similar in patients with and without the metabolic syndrome (0.69 [0.2] vs 0.72 [0.3] U/kg/d).
Figure 2. Prevalence of microvascular complications according to the number of components of the metabolic syndrome.
In the multiple logistic regression analysis, age, body mass index and glycosylated hemoglobin retained a significant and independent association with the presence of the metabolic syndrome (Table 3).
DISCUSSION
This study found a prevalence of the metabolic syndrome in patients with DM1 of 31.9% (31.5% in women and 32% in men). Studies of Americans with DM1 found rates ranging from 21% to 8%, depending on the diagnostic criteria used.22 In Europe, studies such as the FinnDiane study23 found a prevalence of the metabolic syndrome of 39% according to the NCEP-ATP III criteria. Similar data have been reported for the Mediterranean area, where a prevalence of 40.8% was found using the same criteria.24
Of all the components of the metabolic syndrome (independently of glycemia, which was present in all the subjects), the most frequent was hypoalphalipoproteinemia. Persons with DM1 in our area have been found to have low levels of HDL-C (<35 mg/dL in men and <45 mg/dL in women), around 12% in patients with good metabolic control and 20% in those with poor control.25
Of each 3 patients with DM1 and the metabolic syndrome, 2 had abdominal obesity, defined from the abdominal waist circumference. These data agree with those found during the follow-up of the DCCT cohort,26 in which the average weight gain after insulin therapy was 14 kg. Importantly, in the present study no differences were found in the daily insulin requirements between the patients with and without abdominal obesity (0.69 [0.15] vs 0.72 [0.34] U/kg/d), which indicates that factors such as dietary habits, exercise or a family history must be intervening in the development of visceral obesity.
Concerning the repercussion of the metabolic syndrome on chronic complications, the proportion of patients with microangiopathy was clearly greater among those who had this complication. This confirms the results reported in other European series, which have found prevalence rates of the metabolic syndrome reaching 62% in patients with macroalbuminuria, and an odds ratio of 3.75 (95% CI, 2.89-4.85) for diabetic nephropathy in the case of the metabolic syndrome.23 In the present study, the proportion of patients with microangiopathy rose in parallel with the number of components of the metabolic syndrome, reaching 100% in those persons who had all 5 diagnostic criteria.
Studies in a large number of patients with a long-term follow-up have also shown the relation between insulin resistance, the metabolic syndrome and macroangiopathy,12,27,28 an association that was not found in our study. The low number of cases in both groups, the mean age of the patients, the good metabolic control and the relatively short mean duration of the diabetes (16.7 years) may all have contributed to the lack of significant differences in macrovascular complications. In addition, most of these patients were diagnosed after publication of the DCCT study results, and they have therefore followed intensive insulin treatment since their diagnosis, which has been shown to reduce the incidence of severe cardiovascular events by 42% over 20 years.29 Finally, strict criteria have been published for the diagnosis of autoimmune DM1, which exclude young patients with DM2, in whom the prevalence of macroangiopathy on diagnosis may reach 20% given the delay between the onset of the hyperglycemia and the diagnosis,30 due to the close association between DM2, the metabolic syndrome and cardiovascular disease.31
The multiple logistic regression model showed that the degree of blood glucose control, evaluated from the glycosylated hemoglobin, was the most influential variable in the development of the metabolic syndrome, followed by the body mass index and age. Earlier studies found no influence of metabolic control,11 though it is worth noting that the glycosylated hemoglobin in these studies was above 10%, almost 3% higher than in the present study.
Limitations
The limitations of this study derive from its cross-sectional design. Thus, we should recall not only the possible variations over time in the parameters studied but also that the findings only refer to associations, and do not imply causality. The sample size was the result of applying strict criteria for the diagnosis of autoimmune DM1 and excluding patients with a short disease evolution in order to avoid the effects of insulinopenia on the glycosylated hemoglobin and the anthropometric variables. In any event, the baseline characteristics of the patients included in the study were those of the population with DM1 in our setting.32
CONCLUSIONS
The metabolic syndrome is common in patients with DM1, and was present in one third of the patients with diabetes mellitus in our area, particularly the patients who were older, had a higher body mass index and worse metabolic control. The presence of the metabolic syndrome in this group of patients was associated with microvascular complications.
ABBREVIATIONS
HDL: high density lipoproteins.
NCEP-ATP III: National Cholesterol Education Program-Adult Treatment Panel III
SD: standard deviation
UAE: urinary albumin excretion
Correspondence: Dr. J. Pedro-Botet.
Departamento de Medicina. Hospital del Mar. Pg. Marítim, 25-29. 08003 Barcelona. España.
E-mail: 86620@imas.imim.es
Received July 23, 2009.
Accepted for publication December 1, 2009.