Cardiovascular diseases are the leading causes of death in Spain, with ischemic heart disease being the main cause of cardiovascular deaths. Treatment for one common cardiovascular condition, ST-segment elevation acute myocardial infarction (STEMI), has changed considerably in recent years. The recognition that thrombotic occlusion of a coronary artery results in a wavefront of irreversible myocardial cell injury extending from the subendocardium to the subepicardium in a time-dependent fashion led to the introduction of reperfusion therapy for myocardial infarction.1 However, restoration of blood flow to previously ischemic myocardium results in the so-called ischemia/reperfusion (I/R) injury.2
The I/R combination causes numerous and collateral reactions in tissues, culminating in the alteration of essential molecules and organelles in a number of cells including components of the coronary endothelium and myocardium with the recruitment of circulating blood elements, eg, leukocytes and platelets. During the transient ischemia and the period of reperfusion many cells generate byproducts of oxygen that are toxic to the heart. Indeed, the partially reduced oxygen metabolites, both reactive oxygen species (ROS) and reactive nitrogen species (RNS), account for much of the cardiac damage that occurs during I/R injury.2
Solid evidence exists that melatonin influences the cardiovascular system. Melatonin is a multifunctional indolamine that counteracts virtually all pathophysiologic steps and displays significant beneficial actions against ROS/RNS-induced cellular toxicity. This protection is related to melatonin's potent antioxidative and anti-inflammatory properties.3 Melatonin has the capability of scavenging both ROS and RNS, including those formed from peroxynitrite, and blocking transcriptional factors, which induce proinflammatory cytokines. Accumulating evidence suggests that this nontoxic indolamine may be useful either as a sole treatment or in conjunction with other treatments for inhibiting the biohazardous actions of nitrooxidative stress.3
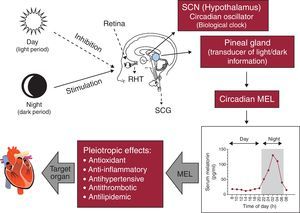
Melatonin and its rhythmMelatonin, an endocrine product of the pineal gland, is formed predominantly during nighttime. Light has an inhibitory effect on pineal melatonin secretion. Melatonin release is synchronized with daylight cycle via a multisynaptic pathway between the eyes and the pineal gland. Light stimulates the retina to modulate the activity of suprachiasmatic nucleus (SCN), the master biological clock. The SCN controls pineal melatonin synthesis and release via the peripheral sympathetic nervous system, which involves synapses in the intermediolateral cell column of the thoracic cord and its projection towards the superior cervical ganglia; postganglionic sympathetic fibers eventually terminate on pinealocytes within the pineal gland. The rate-limiting enzyme in the melatonin synthesis is regulated by norepinephrine and released from sympathetic nerve endings to pinealocytes β1- and α1-adrenoceptors. The concentrations of melatonin in the sera of healthy subjects reach 10-10 to 10-9 mol/l during the night with much lower values being present during the day.3, 4 Mounting evidence documents that melatonin has crucial roles in a variety of cardiovascular pathophysiologic processes: this indoleamine has anti-inflammatory, antioxidant, antihypertensive and possibly antilipidemic functions3 (Figure 1).
Figure 1. Physiological regulation of melatonin by the light/dark environment as detected by the retina. MEL, melatonin; RHT, retino-hypothalamic tract; SCG, superior cervical ganglia; SCN, suprachiasmatic nucleus.
Melatonin also mediates a variety of physiological responses through membrane receptors and nuclear binding sites. The presence of melatonin receptors in cardiomyocytes was suggested by binding studies and immunostaining in chick hearts. Subsequently, MT1 and MT2 membrane receptors have been identified on left ventricle cardiomyocytes of the human heart. The role of melatonin in human ventricular function is still unclear. In isolated rat papillary muscle it possesses antiadrenergic effects and causes a reduction in the force of contractions.5 Likewise, MT1 and MT2 receptors are present in human coronary arteries from pathology samples and also from healthy controls. Animal studies suggest that melatonin has dual effects on the vasculature, depending on the specific receptor type activated, with vasoconstriction occuring after MT1- activation and vasorelaxation after MT2- activation.5
Melatonin and cardiovascular diseaseAtherosclerosis is a chronic vascular disease in which inflammation and oxidative stress are commonly implicated as major causative factors. Early stages of plaque development involve endothelial activation induced by inflammatory cytokines, oxidized low-density lipoprotein, and/or changes in endothelial shear stress.6
Several studies have investigated the antioxidant effect of melatonin on low-density lipoprotein oxidation. Melatonin also has been shown to depress plasma levels of total cholesterol and very low-density lipoprotein cholesterol as well as the low-density lipoprotein cholesterol subfraction in hypercholesterolemic rats.7 Melatonin may exert these effects by increasing endogenous cholesterol clearance. Because of its lipophilic nature, melatonin readily enters the lipid phase of the low-density lipoprotein particles and prevents lipid peroxidation. Dominguez-Rodriguez et al.8 showed an association between nocturnal elevated serum levels of oxidized low-density lipoprotein and reduced circulating melatonin levels in patients with acute myocardial infarction. These findings generally support the notion that melatonin may lower total cholesterol and stimulate high-density lipoprotein levels while reducing the oxidation of low-density lipoprotein, changes that would generally be protective against cardiovascular disease.7
The administration of pharmacological doses of melatonin reduces blood pressure as a consequence of various mechanisms including a direct hypothalamic effect, a lowering of catecholamine levels, relaxation of the smooth muscle wall, and most importantly, its antioxidant properties. There are several reports indicating that melatonin may have a hypotensive effect.7 With a large clinical trial using melatonin as a hypertension treatment, many important questions could be answered, such as the optimum melatonin dose and application regimen and patient selection to achieve greatest possible benefit from melatonin treatment. Based on the accumulated evidence, melatonin seems to be a candidate drug for treatment of hypertension since the number of patients with well-controlled hypertension is alarmingly low worldwide.9
Moreover, there is favorable evidence that the circadian rhythm of melatonin influences insulin secretion and the endocrine pancreas, reduces blood glucose and HbA1c, and restores liver enzymes in diabetic rats. Although little is known about how melatonin reduces plasma glucose in humans, type 2 diabetic patients show a reduced diurnal serum melatonin level and increased pancreatic melatonin-receptors.10 The role of pineal melatonin in preventing or delaying diabetes onset, however, is not clarified, since studies showing beneficial effects of melatonin have been conducted only after onset of the clinical manifestation of diabetes. Nevertheless, a recent comprehensive review has documented a variety of melatonin actions on the physiology of endocrine pancreas that would be expected to reduce the incidence of diabetes.11
Patients with coronary artery disease have low melatonin production rates and blood melatonin concentrations correlate with the severity of the disease, ie, greater reductions in melatonin production are observed in patients with a higher risk of myocardial infarction and/or sudden death. It is uncertain whether low melatonin levels in these patients are the result of melatonin “consumption” caused by scavenging of the elevated free radical production, or represent lower melatonin production, and hence less protection against oxidative stress.3, 4, 12
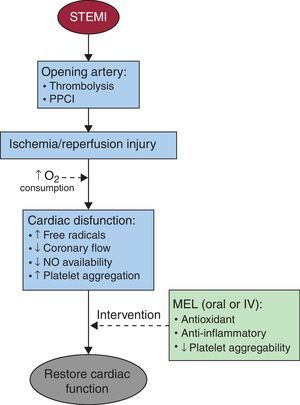
Potential utility of melatonin as an antioxidant during st-elevation myocardial infarctionThere is evidence for cardioprotective effects of melatonin against I/R injury (Figure 2). Melatonin reduces the infarct size/risk area and the incidence of reperfusion arrhythmias. Since ischemia is associated with formation of ROS/RNS from the residual molecular oxygen, the cardioprotective effect is probably associated with melatonin's ability to scavenge free radicals and to induce the expression of antioxidant enzymes.3, 12
Figure 2. Intervention of melatonin for the restoration of cardiac function after an ischemia/reperfusion episode. IV, intravenous; MEL, melatonin; NO, nitric oxide; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation acute myocardial infarction.
Melatonin has scavenging actions at both physiologic and pharmacologic concentrations. Not only melatonin but also several of its metabolites can detoxify free radicals and their derivatives. Melatonin stimulates essential enzymatic intracellular antioxidant enzymes including superoxide dismutases (cytosolic and mitochondrial) and glutathione peroxidase. Moreover, melatonin induces the activity of γ-glutamylcysteine synthetase, thereby stimulating the production of another intracellular antioxidant, glutathione. Of particular interest is the possible role of melatonin as a bioenergetic agent that can improve and maintain mitochondrial function.3, 7
The I/R injury increases oxygen and nitrogen reactant, modifying calcium signaling in platelets, and stimulates aggregation.2 The synthesis of melatonin is not restricted exclusively to the pineal gland but also takes place in extrapineal sources, such as megakaryocytes and platelets.13 A recent study from our group documented a relationship between intraplatelet melatonin content and the “no-reflow” phenomenon in STEMI patients undergoing primary percutaneous coronary intervention. Our data suggest that patients with angiographic no-reflow had lower intraplatelet melatonin levels than patients without this phenomenon, and higher systemic oxidative stress.13 Likewise, recent investigations in STEMI patients undergoing primary percutaneous coronary intervention confirmed a relationship between melatonin concentrations and ischemia-modified albumin, a marker of myocardial ischemia. These data suggest that melatonin acts as a potent antioxidant agent, reducing myocardial damage induced by I/R.14
The available scientific evidence has led our group to carry out a phase II clinical trial (ClinicalTrials.gov no. NCT00640094). We attempt to demonstrate an inhibition of I/R damage after administration of intravenous melatonin in STEMI patients immediately before primary percutaneous coronary intervention.15 Moreover, other authors are testing whether intracoronary injection of melatonin can limit I/R-related myocardial damage (ClinicalTrials.gov no. NCT01172171).
The importance of these studies is emphasized by the fact that melatonin is quickly distributed throughout the organism via exogenous administration (oral, intravenous or subcutaneous). It crosses all morphophysiologic barriers and enters cardiac cells with great ease. Highest intracellular concentrations of melatonin are found at a mitochondrial level. This is especially important, as the mitochondria is a major site of free radical generation and oxidative stress.3, 4, 7
In conclusion, melatonin obviously has a variety of beneficial effects with reference to cardiovascular pathophysiology, including hypertension, diabetes mellitus, dyslipidemia, and I/R injury. Given the severity of these conditions and the uncommonly low toxicity of melatonin, clinical trials using this indole are highly justified. In view of the large amount of positive data that has been already accumulated, we consider melatonin a useful agent to test aspects of cardiovascular pathophysiology. Unless the findings in animal investigations are totally misleading, it seems likely that melatonin will have similar protective effects benefitting the human heart.
Conflicts of interestNone declared.
.
Corresponding author: Servicio de Cardiología, Hospital Universitario de Canarias, Ofra s/n, 38320 La Cuesta, La Laguna, Santa Cruz de Tenerife, Canarias, Spain. adrvdg@hotmail.com