
Clinical and experimental studies have shown that, in patients with reperfused ST-segment elevation myocardial infarction (STEMI), abnormalities in the endothelial monolayer are initiated during ischemia but rapidly intensify upon restoration of blood perfusion to the ischemic area. We aimed to evaluate the effect of serum isolated after revascularization from STEMI patients on the degree of endothelial permeability in vitro, by promoting endothelial cell apoptosis and necrosis in vitro. We also investigated the association between the percentage of serum-induced endothelial cell apoptosis or necrosis in vitro and the extent of cardiovascular magnetic resonance (CMR)-derived parameters of reperfusion injury (edema, hemorrhage, and microvascular obstruction).
MethodsHuman coronary artery endothelial cells were incubated with serum isolated 24hours after revascularization from 43 STEMI patients who underwent CMR and 14 control participants. We assessed the effect of STEMI serum on activation of apoptosis and necrosis, as well as on the permeability and structure of the endothelial monolayer.
ResultsSerum from STEMI patients increased apoptosis (P <.01) and necrosis (P <.05) in human coronary artery endothelial cells and caused increased permeability of the endothelial monolayer in vitro (P <.01), due to enlarged intercellular spaces (P <.05 vs control in all cases). Higher serum-induced necrosis was associated with greater endothelial permeability in vitro (P <.05) and with more extensive CMR-derived indices of reperfusion injury and infarct size.
ConclusionsPostreperfusion serum activates necrosis and apoptosis in endothelial cells and increases the degree of endothelial permeability in vitro. The more potent the necrosis-triggering effect of serum, the more deleterious the consequences in terms of the resulting cardiac structure.
Keywords
After ST-segment elevation myocardial infarction (STEMI), the rapid reopening of the coronary artery causes hemodynamic and oxidative stress known as ischemia-reperfusion injury.1–3 Specifically, abnormalities in endothelial cells (ECs) in reperfused STEMI have been suggested to originate during ischemia but are rapidly intensified upon revascularization.4,5 Reperfusion injuries facilitate capillary obstruction (microvascular obstruction [MVO]), extravasation of the blood content into the interstitium (hemorrhage), and increased water content in the extracellular compartment (interstitial edema).6 These are considered parameters of reperfusion injury and have negative consequences on postinfarction tissue composition. Given that microvessel repair is now considered a central therapeutic target post-STEMI, it is crucial to advance understanding of the mechanisms underlying the pathophysiology of microvascular injury.
In the STEMI scenario, the ischemia-reperfusion insult can activate apoptosis, a programmed cell death mechanism, that can lead to necrosis, resulting in loss of cell membrane integrity and the release of cell detritus.7 Several studies have explored the effect of postreperfusion serum on parameters of endothelial function,8,9 but to our knowledge, there is a paucity of literature on the impact of postreperfusion serum on loss of viability of ECs and on the permeability and structure of the endothelial barrier.
Therefore, the specific aims of our study were as follow: a) to assess in vitro the capacity of serum from STEMI patients to diminish EC viability by promoting apoptotic and necrotic pathways; b) to demonstrate in vitro whether serum from STEMI patients alters the permeability and structure of the endothelial monolayer, and c) to describe the association of serum-induced apoptosis and necrosis in vitro with changes in permeability, and with cardiovascular magnetic resonance (CMR)-derived indices of reperfusion injury (edema, hemorrhage, and MVO) and infarct size.
METHODSStudy populationThe study conformed to the principles outlined in the Declaration of Helsinki, was approved by the local Research Ethics Committee at the Hospital Clinico Universitario de Valencia and written informed consent was obtained from all participants.
Inclusion criteria consisted of patients with a first STEMI defined following current definitions10 who were treated with primary coronary intervention within 12hours of onset of chest pain and who underwent CMR imaging at 1 week and 6 months post-STEMI. We prospectively enrolled 57 consecutive patients discharged between July 2015 and December 2017.
Exclusion criteria were death (n=4), reinfarction (n=2), clinical instability (n=6) during the first 6 months postdischarge and claustrophobia (n=2). The final study group comprised 43 STEMI patients (figure 1 of the supplementary data).
We also recruited a control group matched by age and sex with the study group (table 1 of the supplementary data), composed of 14 patients. In these patients, the presence of any cardiac disease was ruled out through thorough clinical history, physical examination, and echocardiographic study carried out by a clinical cardiologist.
Baseline characteristics and blood samplesBaseline characteristics were prospectively registered in all patients. Patients were managed both in hospital and after discharge by a specific STEMI unit, and current recommendations were strictly followed. Further details of patient characteristics are shown in table 1.
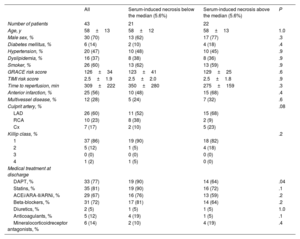
Baseline characteristics of the entire cohort of patients with ST-segment elevation myocardial infarction and of patients with serum-induced necrosis above or below the median
| All | Serum-induced necrosis below the median (5.6%) | Serum-induced necrosis above the median (5.6%) | P | |
|---|---|---|---|---|
| Number of patients | 43 | 21 | 22 | |
| Age, y | 58±13 | 58±12 | 58±13 | 1.0 |
| Male sex, % | 30 (70) | 13 (62) | 17 (77) | .3 |
| Diabetes mellitus, % | 6 (14) | 2 (10) | 4 (18) | .4 |
| Hypertension, % | 20 (47) | 10 (48) | 10 (45) | .9 |
| Dyslipidemia, % | 16 (37) | 8 (38) | 8 (36) | .9 |
| Smoker, % | 26 (60) | 13 (62) | 13 (59) | .9 |
| GRACE risk score | 126±34 | 123±41 | 129±25 | .6 |
| TIMI risk score | 2.5±1.9 | 2.5±2.0 | 2.5±1.8 | .9 |
| Time to reperfusion, min | 309±222 | 350±280 | 275±159 | .3 |
| Anterior infarction, % | 25 (56) | 10 (48) | 15 (68) | .4 |
| Multivessel disease, % | 12 (28) | 5 (24) | 7 (32) | .6 |
| Culprit artery, % | .08 | |||
| LAD | 26 (60) | 11 (52) | 15 (68) | |
| RCA | 10 (23) | 8 (38) | 2 (9) | |
| Cx | 7 (17) | 2 (10) | 5 (23) | |
| Killip class, % | .2 | |||
| 1 | 37 (86) | 19 (90) | 18 (82) | |
| 2 | 5 (12) | 1 (5) | 4 (18) | |
| 3 | 0 (0) | 0 (0) | 0 (0) | |
| 4 | 1 (2) | 1 (5) | 0 (0) | |
| Medical treatment at discharge | ||||
| DAPT, % | 33 (77) | 19 (90) | 14 (64) | .04 |
| Statins, % | 35 (81) | 19 (90) | 16 (72) | .1 |
| ACEi/ARA-II/ARNi, % | 29 (67) | 16 (76) | 13 (59) | .2 |
| Beta-blockers, % | 31 (72) | 17 (81) | 14 (64) | .2 |
| Diuretics, % | 2 (5) | 1 (5) | 1 (5) | 1.0 |
| Anticoagulants, % | 5 (12) | 4 (19) | 1 (5) | .1 |
| Mineralocorticoidreceptor antagonists, % | 6 (14) | 2 (10) | 4 (19) | .4 |
ACEi, angiotensin-converting enzyme inhibitors; ARA-II, angiotensin II receptor antagonists; ARNi, angiotensin receptor neprilysin inhibitors; Cx, circumflex coronary artery; DAPT, dual antiplatelet therapy; GRACE, Global Registry of Acute Coronary Events; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction.
Values are expressed as mean±standard deviation or No. (%).
Blood samples were isolated 24hours after coronary revascularization, centrifuged within the first 2hours after isolation at 800 x g at room temperature for 15minutes and serum was immediately stored at −80°C until further analyses were performed.
Cardiovascular magnetic resonanceCMR (1.5 T unit, Magnetom Sonata; Siemens, Germany) was performed 7 [5-8] days (1-week CMR) and 193 [160-237] days (6-month CMR) after STEMI, in accordance with our laboratory protocol.11,12 All studies were performed and analyzed by 2 cardiologists specialized in CMR with 15 years of experience, using customized software (QMASS MR 6.1.5, Medis, The Netherlands). CMR data were prospectively recorded and immediately included in the database.
Further information on CMR image acquisition and analysis are specified in Methods of the supplementary data.
Human coronary artery endothelial cell cultureA commercial culture of primary human coronary artery endothelial cells (HCAEC) (Lonza, Belgium) was used. According to the manufacturer's information, cells were isolated from the coronary arteries of a healthy donor with cryopreservation on the third passage (500 000 cells). The cells were thawed and cultured in human endothelial growth medium-2 (Lonza, Belgium) supplemented with 10% fetal bovine serum and 100 units/mL of penicillin/100μg/mL of streptomycin (Gibco, Thermo Fisher Scientific, Spain). Cells were routinely grown in sterile conditions in a humidified atmosphere (21% O2) at 37°C and 5% carbon dioxide. The cells were passaged by trypsinization every 2 to 3 days.
When performing further experiments, we used the cells in the sixth passage and a slightly modified ex vivo cell culture assay13 was employed to explore the effect of serum in HCAEC. HCAEC were seeded in gelatin-coated 12-well plates (100 000 cells/mL) and 24hours later (80% confluence) the culture medium was replaced with a modified medium consisting of endothelial growth medium-2 without fetal bovine serum. Next, 10% of STEMI serum drawn 24hours postreperfusion or from control participants was added to the cultures. After 24hours of incubation, the cells were harvested or fixed for further analysis.
Afterward, a multidisciplinary approach was used to evaluate apoptosis and necrosis activation in HCAEC and to assess changes in permeability and structure of the endothelial monolayer in vitro. Further details are included in the methods section of the supplementary data and raw data from all experiments are included in tables 2, 3, 4, and 5 of the supplementary data.
Statistical analysisData were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous normally distributed data are expressed as the mean±standard deviation of the mean and were compared using unpaired Student t-tests. Nonparametric data are expressed as the median [interquartile range] and were compared using the Mann-Whitney U-test. Group percentages were compared using the chi-square test or Fisher exact test, as appropriate. Linear correlations were assessed using Pearson's correlation coefficient or Spearman's rank-order correlation.
Statistical significance was considered for a 2-tailed P <.05. The SPSS statistical package (version 15.0, SPSS Inc, United States) was used throughout.
RESULTSThe baseline characteristics of our final cohort are displayed in table 1. There were no significant differences in baseline characteristics and cardiovascular risk factors between STEMI patients and controls (table 1 of the supplementary data).
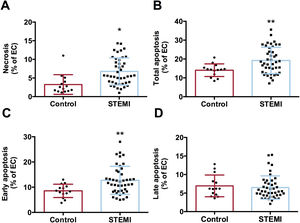
Serum from STEMI patients induces necrosis and apoptosis in endothelial cells in vitroAccording to flow cytometry analysis, an augmented percentage of cells undergoing necrosis (figure 1A) and apoptosis (figure 1B) were detected after treating HCAEC with serum from STEMI patients compared with controls. Specifically, the percentage of cells with early apoptosis increased preferentially in serum from STEMI patients (figure 1C), whereas no differences were detected in late apoptosis (figure 1D) (table 2 of the supplementary data).
Effect of serum from STEMI patients on HCAEC viability. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. The percentages of cells undergoing necrosis (A), apoptosis (B), early apoptosis (C), and late apoptosis (D) determined by flow cytometry were increased after HCAEC treatment with serum from STEMI patients compared with control serum.
EC, endothelial cells; HCAEC, human coronary artery endothelial cells; STEMI, ST-segment elevation myocardial infarction.
*P <.05.
**P <.01 vs controls.
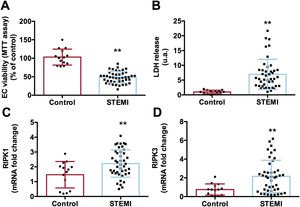
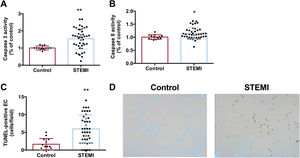
Necrosis activation after treatment with serum from reperfused STEMI patients was confirmed by a drop in cell viability, demonstrated by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay (figure 2A), an augmented release of lactate dehydrogenase into the extracellular medium (figure 2B), and upregulated mRNA expression of receptor interacting serine/threonine-protein kinase 1 and receptor interacting serine/threonine-protein kinase 3 (figure 2C,D, respectively) in HCAEC treated with serum from STEMI patients (table 3 of the supplementary data). To corroborate apoptosis activation, HCAEC incubated with STEMI patient serum displayed not only heightened caspase-3 and caspase-8 activity (figure 3A,B, respectively) but also a higher number of TUNEL-positive cells (figure 3C,D) (table 4 of the supplementary data), which indicated cells with deoxyribonucleic acid fragmentation including apoptotic and necrotic cells.14
Activation of necrosis on HCAEC after treatment with serum from STEMI patients. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. Serum from STEMI patients induced a drop in cell viability as reflected by MTT assay (A) and by augmented LDH release into the extracellular medium (B). Serum from STEMI patients caused augmented mRNA expression of markers of necrosis RIPK1 (C) and RIPK3 (D).
HCAEC, human coronary artery endothelial cells; LDH, lactate dehydrogenase; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; RIPK, receptor interacting serine/threonine-protein kinase; STEMI, ST-segment elevation myocardial infarction.
**P <.01 vs controls.
Activation of apoptosis on HCAEC after treatment with serum from STEMI patients. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. Both caspase-3 (A) and caspase-8 (B) activity and the number of TUNEL-positive cells (C) were intensified in HCAEC treated with serum from STEMI patients compared with controls. Representative images from TUNEL staining performed on HCAEC treated with serum from controls or STEMI patients (D).
HCAEC, human coronary artery endothelial cells; STEMI, ST-segment elevation myocardial infarction.
*P <.05.
**P <.01 vs controls.
All these results indicated that serum isolated 24hours postreperfusion from STEMI patients had a deleterious effect on ECs by activating apoptosis and necrosis in vitro.
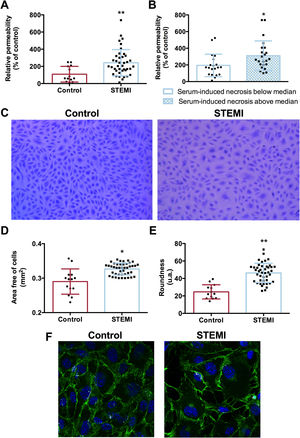
Serum from STEMI patients enhanced the permeability of the endothelial monolayerWe examined whether serum from STEMI patients could also affect the structure and function of the endothelial barrier, performing an in vitro permeability assay using FITC-dextran conjugate. Compared with serum from controls, serum from STEMI patients showed increased FITC-dextran migration through the vascular barrier (figure 4A).
Impact of serum from STEMI patients on the endothelial barrier. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. Increased permeability was detected in HCAEC treated with serum from STEMI compared with controls (A). Patients whose serum induced an above median percentage of HCAEC undergoing necrosis in vitro had a higher degree of permeability in vitro (B). Representative pictures of the endothelial barrier after incubation with serum from controls and STEMI patients (C). Serum from STEMI patients induced an increase in the area free of cells within the endothelial barrier (D) as well as high endothelial cell roundness (E). The endothelial barrier treated with serum from controls or STEMI patients (F) was incubated with a mouse antihuman VE-cadherin antibody (1:250) and immunoreactivity was visualized using Alexa Fluor 488 (VE-cadherin, green) secondary antibody under confocal microscopy. Nuclei were stained with DAPI (blue).
HCAEC, human coronary artery endothelial cells; STEMI, ST-segment elevation myocardial infarction; VE, vascular endothelial.
*P <.05
**P <.01 vs controls.
Next, cells were stained and photographed to morphometrically assess structural changes in the endothelial monolayer. Although the number of cells per field was similar in the 2 groups, serum from STEMI patients significantly increased the space between cells (figure 4D) as well as their roundness (figure 4E) (table 5 of the supplementary data). Vascular endothelial-cadherin was also visualized using confocal microscopy and changes in the endothelial barrier were also detected (figure 4F). Overall, serum from STEMI patients also produced a deleterious effect in terms of intensifying vascular permeability by enlarging intercellular spaces.
Last, STEMI patients were dichotomized according to the median percentage of HCAEC undergoing total apoptosis (18.4%) and necrosis (5.6%) on flow cytometry analysis after incubation with patient serum. Table 1 shows the baseline characteristics of STEMI patients with an above or below average median percentage of HCAEC undergoing necrosis in vitro.
Patients whose serum induced a high percentage of necrotic cells in flow cytometry analysis also displayed a more permeable endothelial barrier using an in vitro vascular permeability assay (P <.05, figure 4B). Conversely, no association was found between the percentage of apoptotic cells and degree of vascular permeability.
Relationship between serum-induced necrosis and CMR-derived parameters of reperfusion injuryWe evaluated the association between serum-induced necrosis and apoptosis in vitro by flow cytometry analysis, with the resulting cardiac structure evaluated by CMR studies at 1 week and 6 months after reperfusion (table 2).
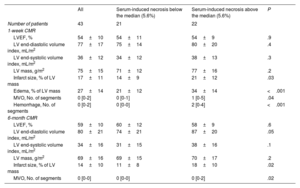
CMR characteristics of the entire cohort of patients with ST-segment elevation myocardial infarction and of patients with serum-induced necrosis above or below the median
| All | Serum-induced necrosis below the median (5.6%) | Serum-induced necrosis above the median (5.6%) | P | |
|---|---|---|---|---|
| Number of patients | 43 | 21 | 22 | |
| 1-week CMR | ||||
| LVEF, % | 54±10 | 54±11 | 54±9 | .9 |
| LV end-diastolic volume index, mL/m2 | 77±17 | 75±14 | 80±20 | .4 |
| LV end-systolic volume index, mL/m2 | 36±12 | 34±12 | 38±13 | .3 |
| LV mass, g/m2 | 75±15 | 71±12 | 77±16 | .2 |
| Infarct size, % of LV mass | 17±11 | 14±9 | 21±12 | .03 |
| Edema, % of LV mass | 27±14 | 21±12 | 34±14 | <.001 |
| MVO, No. of segments | 0 [0-2] | 0 [0-1] | 1 [0-5] | .04 |
| Hemorrhage, No. of segments | 0 [0-2] | 0 [0-0] | 2 [0-4] | <.001 |
| 6-month CMR | ||||
| LVEF, % | 59±10 | 60±12 | 58±9 | .6 |
| LV end-diastolic volume index, mL/m2 | 80±21 | 74±21 | 87±20 | .05 |
| LV end-systolic volume index, mL/m2 | 34±16 | 31±15 | 38±16 | .1 |
| LV mass, g/m2 | 69±16 | 69±15 | 70±17 | .2 |
| Infarct size, % of LV mass | 14±10 | 11±8 | 18±10 | .02 |
| MVO, No. of segments | 0 [0-0] | 0 [0-0] | 0 [0-2] | .02 |
CMR, cardiovascular magnetic resonance; LV, left ventricular; LVEF, left ventricular ejection fraction; MVO, microvascular obstruction.
MVO and hemorrhage are expressed as median [percentile 25 – percentile 75]. All other variables are expressed as mean±standard deviation.
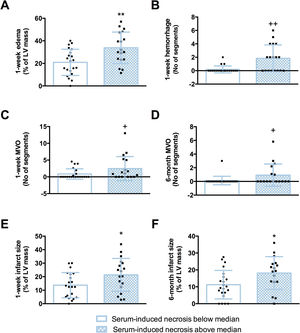
Patients whose serum caused an augmented percentage (above the median) of HCAEC to undergo necrosis in vitro had more extensive CMR-derived edema (figure 5A), hemorrhage (figure 5B), and MVO (figure 5C) 1 week after myocardial infarction (table 2, figure 1 of the supplementary data). At the 6-month CMR study, hemorrhage and edema had almost vanished in all patients, while patients with a percentage of HCAEC undergoing necrosis above the median displayed higher values of MVO in the chronic phase (figure 5D) (table 2, figure 5). Finally, a positive association was also detected between the percentage of HCAEC undergoing necrosis in vitro and the extent of the infarct size as derived by 1-week and 6-month CMR studies (figure 5E and figure 4F, respectively) (table 2, figure 2 of the supplementary data). In contrast, the percentage of cells undergoing apoptosis in vitro was not associated with these CMR-derived indices when evaluated at either 1 week or 6 months (tables 6 and 7 of the supplementary data).
Association between serum-induced necrosis and CMR-derived indices of cardiac structure after STEMI. STEMI patients (n=43) were dichotomized by the median percentage of HCAEC undergoing necrosis (5.6%) in flow cytometry analysis after incubation with serum. Patients whose serum caused an augmented percentage of HCAEC undergoing necrosis in vitro (above the median value) had extensive edema (A), hemorrhage (B), MVO (C), and infarct size (E) at acute phase (1 week postrevascularization), as well as higher MVO (D) and infarct size (F) at chronic phase (6 months).
CMR, cardiovascular magnetic resonance; HCAEC, human coronary artery endothelial cells; LV, left ventricle; MVO, microvascular obstruction; STEMI, ST-segment elevation myocardial infarction.
+P <.05.
++P <.01 vs serum-induced necrosis below median, analyzed using Mann–Whitney U-test.
*P <.05.
**P <.01 vs serum-induced necrosis below median, analyzed using unpaired Student's t-test.
Collectively, necrosis, but not apoptosis, induced by serum from STEMI patients in vitro was associated with the resulting cardiac structure at both the acute and chronic phases after myocardial infarction (figure 6).
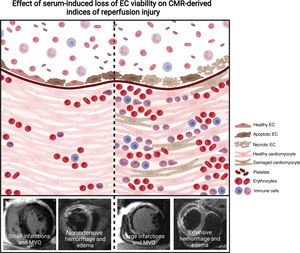
Central illustration. Treatment of HCAEC with serum isolated from STEMI patients induces a loss of cell viability by activating apoptosis and necrosis, as well as disruption in the structure and function of the endothelial monolayer.
Left panel represents those patients with a higher percentage of endothelial cells undergoing necrosis after treatment with serum isolated from STEMI patients 24hours postrevascularization. This subgroup of patients displays the highest degree of permeability of the endothelial monolayer in vitro, leading to increased migration of plasma, erythrocytes, and leukocytes into the cardiac tissue. These 3 phenomena are related to the occurrence of CMR-derived MVO, intramyocardial hemorrhage and edema (indices of reperfusion injury). Collectedly, the disruption of the cell membrane induced after necrosis activation on endothelial cells might be associated with an increase in vascular permeability, which ultimately will result in deleterious cardiac structure indices.
Right panel represents those patients with a lower percentage of endothelial cells undergoing necrosis in vitro after incubation of postreperfusion sera. These patients show a more preserved endothelial barrier, which limits the migration of plasma, erythrocytes, and leukocytes to the myocardium. Consequently, their resulting cardiac structure is less compromised than that of patients whose sera provokes a higher grade of necrosis. Image created by BioRender.
CMR, cardiovascular magnetic resonance; EC, endothelial cells; HCAEC, human coronary artery endothelial cells; MVO, microvascular obstruction; STEMI, ST-segment elevation myocardial infarction.
Prompt and complete restoration of epicardial coronary flow after STEMI is unquestionably essential to limit infarct size, improve long-term ventricular function, and crucially, reduce adverse events during follow-up.1,15
The effect of reperfusion injury on myocardial circulation has been extensively addressed in both clinical and experimental scenarios.4–6,8 A recent study explored changes in the endothelial monolayer at the epicardial level after ischemia-reperfusion injury.5 Although mild abnormalities in the ECs were detected during ischemia, they dramatically increased after reperfusion, as reflected by an almost complete absence of CD31-positive cells.5 Consistent with these data, a decline in microvessel density, and an abnormal ultrastructure in ECs from small capillaries also occur soon after reperfusion.4,6
This trend was maintained in STEMI patients. Despite complete restoration of epicardial flow, a considerable number of patients showed hypoperfused areas within the infarct region.16 This phenomenon, known as MVO, has deleterious consequences in terms of the resulting cardiac structure and prognosis.15,17 Since microvessel repair could become a central therapeutic target post-STEMI18 there is a need to advance understanding of the mechanisms underlying the pathophysiology of microvascular injury, in which ECs are the main actors.
Apoptosis and necrosis on endothelial cells following STEMIIn the ischemia and reperfusion process, in vitro and in vivo experiments have shown that ECs resisted prolonged periods (24-48hours) of hypoxia19,20 by triggering protective mechanisms.20 However, a previous study in a small patient cohort (n=20) suggested that serum from STEMI patients isolated after coronary revascularization induced apoptosis in ECs in vitro as evaluated by flow cytometry. Specifically, this investigation concluded that a gradual augmentation of ECs apoptosis was detected in sera incubations from prereperfusion to 30 days, peaking a few hours after reperfusion.13 In this regard, a recent study performed in 153 patients with acute coronary syndrome found an increased number of endothelial-derived microvesicles related to apoptosis, especially in patients with STEMI.21
Ischemia-reperfusion insult has traditionally been linked to necrosis and apoptosis in cardiomyocytes.7 Briefly, apoptosis is characterized by cell shrinkage and chromatin condensation, whereas necrosis provokes destruction of cell membranes and ruptured mitochondria. Although loss of EC viability after STEMI has been demonstrated,4,22 the effect of serum isolated soon after reperfusion on endothelium viability per se has barely been explored. Accordingly, our first aim was to shed further light on apoptosis and necrosis activation in ECs treated with serum isolated 24hours postreperfusion from STEMI patients.
We confirm that serum isolated 24hours postrevascularization produces negative effects in ECs at different subcellular levels: in mitochondria as reflected in 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay, and in cell membrane by increased release of lactate dehydrogenase into the extracellular medium. Moreover, flow cytometry analysis using the gold-standard technique for assessing cell viability, double-staining for FITC-annexin-V binding and propidium iodide, also confirmed that serum from STEMI patients dramatically activates apoptosis and necrosis in these cells. The activation of both phenomena was also reinforced by upregulation of solid markers of these 2 pathways in HCAEC. Postreperfusion serum seems to exert a dual effect on ECs. It has been reported to activate angiogenesis on coronary ECs via rapid augmentation of proangiogenic factors soon after the onset of ischemia.8,9 Furthermore, these data indicate that serum might also exert a negative impact on ECs beyond the effect of lack of oxygen and nutrients during ischemia and rapid restoration of coronary flow.
Endothelial barrier function and structure after STEMIVascular integrity can be compromised by several phenomena. Our results demonstrate the deleterious effect of postreperfusion serum on EC viability, but to our knowledge there are very few in vitro studies describing the impact of serum from STEMI patients on the proper function and structure of the vascular barrier.
According to our data, serum from STEMI patients induced hyperpermeability, as reflected by increased FITC-dextran leakage through the endothelial barrier. As well as evaluating function, we also checked the structure of the endothelial barrier by light microscopy and confocal microscopy using antivascular endothelial-cadherin antibody, an adhesion molecule located at junctions between ECs. A significant augmentation of intercellular spaces was detected after incubation with serum isolated postreperfusion from STEMI patients compared with controls. An increased cell roundness was also detected in STEMI-serum HCAEC-treated cells. A plausible explanation is that the loss of EC viability promotes a differential cell state in growing cells, resulting in an enlarged cell roundness.
Reperfusion injury has previously been linked to disruption in EC lining via different mechanisms.4–6 Boosting intracellular calcium after coronary revascularization activates the contractile elements in ECs, resulting in the formation of intercellular gaps, which increase their permeability to large molecules.23 Another study performed in rats indicated that reperfusion has an even higher impact on endothelial junctions (key proteins in protecting endothelial integrity) than prolonged ischemia times.4
Our study represents a different scenario, however, in which only the role of serum from STEMI patients can disrupt endothelial permeability. Although beyond the scope of our study, further research should explore whether blockage/promotion of these molecules in vitro might reduce serum-induced vascular hyperpermeability in vitro.
Association between loss of endothelial cell viability and CMR-derived indices of reperfusion injuryAbnormalities in the integrity of the endothelial monolayer following reperfusion leads to extravasation of blood content and plasma fluid into the infarcted myocardium.4,6 Both cause external compression of the capillaries, hindering an adequate blood supply to the infarcted myocardium, which ultimately results in MVO.6 These 3 phenomena can be considered indices of reperfusion injury, which appear a few hours after coronary revascularization and have been linked to adverse patient outcomes. Consequently, we next sought to evaluate the contribution of EC apoptosis and necrosis to the pathophysiology of the above 3 three indices of reperfusion injury.
Our results showed an association between serum-induced necrosis in ECs in vitro and the degree of endothelial monolayer permeability. In line with these results, necrosis in a high percentage of ECs after treatment with serum isolated from STEMI patients after reperfusion also correlated with more extensive CMR-derived edema, hemorrhage, and MVO. These data reveal that together with disruption in intercellular junctions,4 activation of necrosis, a form of irreversible cell injury characterized by cell membrane rupture, could also participate in promoting microvascular injury via increased migration of plasma, erythrocytes, and leukocytes, leading to a more compromised cardiac structure after STEMI. Conversely, no association between these indices and activation of apoptosis was detected, likely because apoptosis has a lesser impact on organ structure than necrosis. Last, although many factors (ie, ischemia-reperfusion injury, atherosclerotic plaque dislodgements during percutaneous intervention, patients’ baseline comorbidities) participate in the pathophysiology of MVO,6,16 these data pointed out that sera itself is an extra factor exerting a negative impact on coronary ECs that results in worse cardiac structure.
LimitationsThis is an in vitro study, focused primarily on the correlation between necrosis and damage to the endothelial monolayer due to postreperfusion serum isolated from STEMI patients. The isolation of blood samples drawn from the coronary beds rather than peripheral samples and the use of human microvascular ECs instead of HCAEC would be better to simulate the in vivo situation.
Regarding cell roundness, the differential cell state in growing cells after incubation with STEMI patients’ sera could influence this index.
It would also have been useful to explore the mechanisms underlying serum-induced HCAEC loss and to research the effect of serum on endothelial function in greater depth. Finally, these results require confirmation in experimental and clinical models, as the cells were not subjected to ischemia-reperfusion injury, one of the main sources of endothelial injury postmyocardial infarction.
CONCLUSIONSSerum from STEMI patients induced a loss of EC viability by activating apoptosis and necrosis in vitro, and also increased EC permeability by enlarging intercellular spaces. Serum-induced necrosis, but not apoptosis, in vitro on HCAEC was linked to the degree of endothelial monolayer permeability, as well as to CMR-derived parameters of reperfusion injury (edema, hemorrhage, and MVO) and infarct size.
FUNDINGThis work was supported by grants from Instituto de Salud Carlos III and Fondos Europeos de Desarrollo Regional (FEDER) (grant numbers PI20/00637 and CIBERCV16/11/00486, postgraduate contract CM21/00175 to V. Marcos-Garcés), by Conselleria de Educación – Generalitat Valenciana (PROMETEO/2021/008 to V. Bodí. and CIGE/2022/26 to V. Marcos-Garcés). J. Gavara acknowledges financial support from the Agencia Estatal de Investigación (grant FJC2020-043981-I/AEI/10.13039/501100011033).
AUTHORS’ CONTRIBUTIONSC. Ríos-Navarro: conceived and designed the analysis; collected the data; performed formal analysis; wrote the original draft; revised and edited the final version. J. Gavara: collected the data; performed formal analysis; wrote the original draft; revised and edited the final version. E. de Dios: collected the data; revised and edited the final version. N. Pérez-Solé: collected the data; revised and edited the final version. T. Molina-García: collected the data; revised and edited the final version. V. Marcos-Garcés: collected the data; revised and edited the final version. A. Ruiz-Saurí: collected the data; revised and edited the final version. A. Bayés-Genís: collected the data; revised and edited the final version. F. Carrión-Valero: collected the data; revised and edited the final version. F. J Chorro: financial support; revised and edited the final version. V. Bodí: conceived and designed the analysis; performed formal analysis; financial support; wrote the original draft; revised and edited the final version.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
Abnormalities in the endothelial monolayer following STEMI are initiated during ischemia but rapidly intensity upon coronary revascularization. Reperfusion injuries facilitate MVO, hemorrhage, and edema. However, to our knowledge, little research has been conducted into the impact of postreperfusion serum on loss of viability of ECs and on the permeability and structure of the endothelial barrier.
WHAT DOES THIS STUDY ADD?Postreperfusion sera activate necrosis and apoptosis in ECs and increase the degree of endothelial permeability in vitro. Indeed, necrosis in a high percentage of ECs after treatment with serum isolated from STEMI patients after reperfusion also correlated with more extensive CMR-derived cardiac structure. These data reveal that activation of necrosis, a form of irreversible cell injury characterized by cell membrane rupture, could also promote microvascular injury, resulting in a more compromised cardiac structure post-STEMI.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.08.004


![Activation of necrosis on HCAEC after treatment with serum from STEMI patients. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. Serum from STEMI patients induced a drop in cell viability as reflected by MTT assay (A) and by augmented LDH release into the extracellular medium (B). Serum from STEMI patients caused augmented mRNA expression of markers of necrosis RIPK1 (C) and RIPK3 (D). HCAEC, human coronary artery endothelial cells; LDH, lactate dehydrogenase; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; RIPK, receptor interacting serine/threonine-protein kinase; STEMI, ST-segment elevation myocardial infarction. **P <.01 vs controls. Activation of necrosis on HCAEC after treatment with serum from STEMI patients. HCAEC were incubated with control serum (n=14) or STEMI patient serum (n=43) isolated 24hours after reperfusion. Serum from STEMI patients induced a drop in cell viability as reflected by MTT assay (A) and by augmented LDH release into the extracellular medium (B). Serum from STEMI patients caused augmented mRNA expression of markers of necrosis RIPK1 (C) and RIPK3 (D). HCAEC, human coronary artery endothelial cells; LDH, lactate dehydrogenase; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; RIPK, receptor interacting serine/threonine-protein kinase; STEMI, ST-segment elevation myocardial infarction. **P <.01 vs controls.](https://static.elsevier.es/multimedia/18855857/0000007700000003/v1_202402270529/S1885585723002384/v1_202402270529/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6InJoUnU1ZUVpU0JGZHA4U2JlT0Mybnc9PSIsInZhbHVlIjoiUTBYYkJubWZKWkJ2bGpCcldjU09zbXUvMTJ4UGdMSTFicXROdWdUZWJTcz0iLCJtYWMiOiJkM2I1YjAxM2VhZDA4OGFhYjYxYjY3ZGU4NWRkMjA5OWVkNmFmODMyNWE2NzlmNzIwYjk1NGI1YTIxMzk1M2FjIiwidGFnIjoiIn0=)



