Mild therapeutic hypothermia (MTH) has been linked to an increased risk of both thrombotic and bleeding events in comatose survivors after out-of-hospital cardiac arrest (OHCA) and in patients with acute coronary syndrome (ACS) after OHCA who undergo percutaneous coronary intervention (PCI).1,2 Controversially, MTH is associated with an increased risk of stent thrombosis (ST).3 The postcardiac arrest (CA) state by itself might increase the thrombotic/bleeding risk regardless of MTH, so that the clinical effects of MTH by itself have been debated.3,4 Thus, the aim of this study was to assess the incidence of thrombotic/bleeding events in patients with ACS after an OHCA, depending on whether they received MTH.
This was a single-center observational study. We screened consecutive patients admitted to our hospital between 2005 and January 2016 with ACS and OHCA undergoing PCI. Since 2010, the MTH protocol has been used in our center for comatose patients after OHCA of presumed cardiac cause regardless of the initial rhythm. We compared outcomes in these patients with those in other patients not undergoing MTH from 2005 to 2009.
Exclusion criteria included OHCA patients with contraindications for MTH (pregnancy, temperature on admission < 30°C, the use of coumadin products, previous use of a fibrinolytic, suspected or known acute intracranial hemorrhage, or stroke), and patients who died before the index procedure. The study was approved by the Ethics Committee of our center (retrospective data collection).
All surviving OHCA patients with high suspicion of ACS (electrocardiogram changes, initial shockable rhythm, or previous chest pain) were admitted to the cardiac catheterization laboratory. Patients were treated with aspirin and heparin. PCI was attempted if there was an acute coronary atherothrombotic lesion. A loading dose of P2Y12 inhibitors was crushed and administered by nasogastric tubing immediately after PCI. The loading dose was followed by a maintenance dose. Since 2010, patients received MTH to 33°C according to the local intensive cardiac unit protocol.
The primary endpoint was the occurrence of thrombotic events including definite and probable ST, as well as the incidence of bleeding events according to the Bleeding Academic Research Consortium criteria during hospitalization.
From 2005 to 2016, 204 patients were treated after OHCA in the intensive cardiac unit. Of these, 145 had an ACS. From 2005 to 2009, 40 patients (38%) did not received MTH, whereas from 2010 to 2016, 105 patients (62%) were treated with MTH. Baseline clinical and procedural characteristics are presented in Table 1. During hospitalization, no differences among groups were seen for thrombotic events, any or major bleeding, and mortality (Table 2). All ST occurred in patients receiving clopidogrel. In those patients who received clopidogrel there were no significant differences in the incidence of ST with MTH: 14% vs no MTH 8.8%; P = .52. In a multivariable logistic regression model incorporating covariates such as MTH, thromboaspiration, stent type, and clopidogrel use, MTH was not a significant predictor of ST with an adjusted odds ratio, 1.1; 95% confidence interval, 0.24-4,95; P = .89.
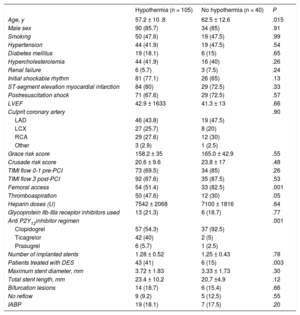
Baseline Clinical and Procedural Characteristics
| Hypothermia (n = 105) | No hypothermia (n = 40) | P | |
|---|---|---|---|
| Age, y | 57.2 ± 10 .8 | 62.5 ± 12.6 | .015 |
| Male sex | 90 (85.7) | 34 (85) | .91 |
| Smoking | 50 (47.6) | 19 (47.5) | .99 |
| Hypertension | 44 (41.9) | 19 (47.5) | .54 |
| Diabetes mellitus | 19 (18.1) | 6 (15) | .65 |
| Hypercholesterolemia | 44 (41.9) | 16 (40) | .26 |
| Renal failure | 6 (5.7) | 3 (7.5) | .24 |
| Initial shockable rhythm | 81 (77.1) | 26 (65) | .13 |
| ST-segment elevation myocardial infarction | 84 (80) | 29 (72.5) | .33 |
| Postresuscitation shock | 71 (67.6) | 29 (72.5) | .57 |
| LVEF | 42.9 ± 1633 | 41.3 ± 13 | .66 |
| Culprit coronary artery | .90 | ||
| LAD | 46 (43.8) | 19 (47.5) | |
| LCX | 27 (25.7) | 8 (20) | |
| RCA | 29 (27.6) | 12 (30) | |
| Other | 3 (2.9) | 1 (2.5) | |
| Grace risk score | 158.2 ± 35 | 165.0 ± 42.9 | .55 |
| Crusade risk score | 20.6 ± 9.6 | 23.8 ± 17 | .48 |
| TIMI flow 0-1 pre-PCI | 73 (69.5) | 34 (85) | .26 |
| TIMI flow 3 post-PCI | 92 (87.6) | 35 (87.5) | .53 |
| Femoral access | 54 (51.4) | 33 (82.5) | .001 |
| Thromboaspiration | 50 (47.6) | 12 (30) | .05 |
| Heparin doses (U) | 7542 ± 2068 | 7100 ± 1816 | .64 |
| Glycoprotein IIb-IIIa receptor inhibitors used | 13 (21.3) | 6 (18.7) | .77 |
| Anti P2Y12inhibitor regimen | .001 | ||
| Clopidogrel | 57 (54.3) | 37 (92.5) | |
| Ticagrelor | 42 (40) | 2 (5) | |
| Prasugrel | 6 (5.7) | 1 (2.5) | |
| Number of implanted stents | 1.28 ± 0.52 | 1.25 ± 0.43 | .78 |
| Patients treated with DES | 43 (41) | 6 (15) | .003 |
| Maximum stent diameter, mm | 3.72 ± 1.83 | 3.33 ± 1.73 | .30 |
| Total stent length, mm | 23.4 ± 10.2 | 20.7 ±4.9 | .12 |
| Bifurcation lesions | 14 (18.7) | 6 (15.4) | .66 |
| No reflow | 9 (9.2) | 5 (12.5) | .55 |
| IABP | 19 (18.1) | 7 (17.5) | .20 |
DES, drug-eluting stent; IABP, intra-aortic balloon pump; LAD, left descending artery; LCX, left circumflex artery; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis In Myocardial Infarction.
Data are expressed as No. (%) or mean ± standard deviation.
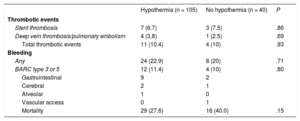
Outcomes
| Hypothermia (n = 105) | No hypothermia (n = 40) | P | |
|---|---|---|---|
| Thrombotic events | |||
| Stent thrombosis | 7 (6.7) | 3 (7.5) | .86 |
| Deep vein thrombosis/pulmonary embolism | 4 (3.8) | 1 (2.5) | .69 |
| Total thrombotic events | 11 (10.4) | 4 (10) | .93 |
| Bleeding | |||
| Any | 24 (22.9) | 8 (20) | .71 |
| BARC type 3 or 5 | 12 (11.4) | 4 (10) | .80 |
| Gastrointestinal | 9 | 2 | |
| Cerebral | 2 | 1 | |
| Alveolar | 1 | 0 | |
| Vascular access | 0 | 1 | |
| Mortality | 29 (27.6) | 16 (40.0) | .15 |
BARC, Bleeding Academic Research Consortium.
Data are expressed as No. (%).
The incidence of ST in OHCA patients has been poorly studied with variable results ranging from 1.4 to 45.5% in small series.3 This study shows a high incidence in ST in OHCA patients after PCI (6.8% overall). In most studies, the relationship between MTH and ST is studied without an OHCA patient control group not undergoing MTH.
Our study did not show a higher incidence of thrombotic events in patients under MTH compared with the control group, similar to a previous report3 suggesting that the post-CA state by itself could be the trigger for ST. In the present study, the new P2Y12 inhibitors were used more often in the last years. Previously, our group has reported a reduction in the incidence of ST in patients with ACS after OHCA and MTH with the use of ticagrelor compared with clopidogrel.5 The present study shows high rates of any and major bleeding events (11%) in ACS patients after OHCA undergoing PCI. There were no differences depending on the use of MTH. The bleeding rate in patients is in keeping with that of previously published studies in OHCA patients.2 Thus, the clinical effects of MTH in addition to the post-CA state remain controversial. The comatose state of patients hampers the administration of oral P2Y12 inhibitors, and multiorgan failure can result in delayed absorption and metabolism of antiplatelet drugs and a subsequent increased risk of ST. OHCA patients often present with acidosis, tissue damage, and high levels of intrinsic or therapeutically administered catecholamines that might facilitate hemorrhagic complications.
The present study has several limitations. It is a single-center nonrandomized study that includes patients treated in a long period and heterogeneous groups with changes in treatment protocols (MTH, thromboaspiration, radial access, new P2Y12 inhibitors, drug-eluting stents). The MTH group showed a reduction in mortality. These patients were those included in the last years of the study and probably benefited from advances in the treatment. Although this reduction was not statistically significant, this was probably due to the small sample size of this study.
In conclusion, the incidence of thrombotic/bleedings events in this study was high in patients with ACS undergoing PCI after an OHCA. The use of MTH was not associated with an increased risk of thrombotic/bleeding events. The results are hypothesis-generating and there is a need for further randomized data.
.