To assess the effectiveness of direct oral anticoagulants vs vitamin K antagonists in real-life patients with atrial fibrillation.
MethodsA systematic review was performed according to Cochrane methodological standards. The results were reported according to the PRISMA statement. The ROBINS-I tool was used to assess risk of bias.
ResultsA total of 27 different studies publishing data in 30 publications were included. In the studies with a follow-up up to 1 year, apixaban (HR, 0.93; 95%CI, 0.71-1.20) and dabigatran (HR, 0.95; 95%CI, 0.80-1.13) did not significantly reduce the risk of ischemic stroke vs warfarin, whereas rivaroxaban significantly reduced this risk (HR, 0.83; 95%CI, 0.73-0.94). Apixaban (HR, 0.66; 95%CI, 0.55-0.80) and dabigatran (HR, 0.83; 95%CI, 0.70-0.97) significantly reduced the major bleeding risk vs warfarin, but not rivaroxaban (HR, 1.02; 95%CI, 0.95-1.10), although with a high statistical heterogeneity among studies. Apixaban (HR, 0.56; 95%CI, 0.42-0.73), dabigatran (HR, 0.45; 95%CI, 0.39-0.51), and rivaroxaban (HR, 0.66; 95%CI, 0.49-0.88) significantly reduced the risk of intracranial bleeding vs warfarin. Reduced doses of direct oral anticoagulants were associated with a slightly better safety profile, but with a marked reduction in stroke prevention effectiveness.
ConclusionsData from this meta-analysis suggest that, vs warfarin, the stroke prevention effectiveness and bleeding risk of direct oral anticoagulants may differ in real-life patients with atrial fibrillation.
Keywords
Atrial fibrillation (AF), the most common arrhythmia in clinical practice,1 is associated with a markedly increased risk of stroke. AF-associated stroke has a higher risk of death, disability, and recurrence.2 A cornerstone in the prevention of thromboembolic complications in patients with AF is anticoagulation.3 Although vitamin K antagonists (VKAs) are associated with a 60% reduction in the risk of stroke in AF patients,4 they have many disadvantages that have limited their use in clinical practice, including a narrow therapeutic window, multiple interactions with other drugs and foods, and the need for frequent monitoring of anticoagulant activity and for dosage adjustments.5
Direct oral anticoagulants (DOACs) overcome most of these limitations. In addition, a number of phase III clinical trials have shown that DOACs are at least as effective as VKAs for the prevention of stroke or systemic embolism, but have a better safety profile, particularly regarding the risk of intracranial bleeding.6–8
The introduction of DOACs to routine clinical practice has been associated with improved rates of overall oral anticoagulation use in patients with AF.9 Due to strict clinical trial inclusion and exclusion criteria and closer patient follow-up, patients with AF participating in clinical trials frequently differ from those in clinical practice. Thus, the information reported in randomized clinical trials is not always valid for real-life patients.10,11 In the last few years, many claims database studies have analyzed the use of DOACs in AF in clinical practice. However, discrepancies between data sources, the statistical analysis approach, and patient characteristics can lead to conflicting results. In this context, a comprehensive analysis of all published information on the use of DOACs in patients with AF vs VKAs is of great interest. The aim of this systematic review and meta-analysis was to assess the effectiveness and safety of DOACs (apixaban, dabigatran, and rivaroxaban) vs VKAs in real-life patients with nonvalvular AF.
METHODSA systematic review was performed according to Cochrane methodological standards.12 Results were reported according to the PRISMA statement.13 The review protocol is detailed in the Methods section of the supplementary material. C. Escobar and J. Martí-Almor selected the articles to be included. If there was disagreement, a third researcher, A. Pérez Cabeza, was consulted. All authors were responsible for data extraction. M.J. Martínez-Zapata was responsible for data analysis. All authors were involved in writing the manuscript.
Eligibility CriteriaWe included observational controlled studies in patients diagnosed with nonvalvular AF comparing any DOAC (apixaban, dabigatran, or rivaroxaban; any dose) vs VKAs (focusing on warfarin).
Only studies that reported rates and effect measures (hazard ratios [HRs]) were included in this meta-analysis. Inclusion was limited to observational studies (either prospective or retrospective) reporting on any of the abovementioned efficacy and safety outcomes from routinely collected health data. Studies with national or regional registries or registries covering a large population across multiple sites were included. Single-center studies using local registries were excluded unless they had more than 1000 patients. For studies that used the same registry and were conducted in the same period (or with substantial overlap), the most complete publication was selected. Only when the degree of overlap between studies was minimal were all publications included.
OutcomesThe outcomes of interest are detailed in the protocol described in the Methods section of the supplementary material. We prioritized ischemic stroke and the composite end point of stroke/systemic embolism for efficacy and major and intracranial bleeding for safety. All outcomes were assessed according to follow-up duration (up to 1 year, as well as longer) and anticoagulant dose (standard vs reduced).
Search StrategyTo retrieve studies of interest, a MEDLINE (via PubMed) and EMBASE (via Ovid) search was performed up to March 2017. Search algorithms adapted to the requirements of each database were designed; these algorithms included a combination of controlled vocabulary search terms and filters to retrieve clinical trials and cohort studies (). In addition, the bibliography sections of the retrieved eligible studies were further used to identify additional relevant studies. No temporal or linguistic limitations were applied to the searches.
For the study selection, search results were screened based on the title and abstract independently. Full-text versions of the articles deemed to be eligible were retrieved and eligibility was independently confirmed based on the inclusion criteria. Disagreements were resolved by reaching consensus or by consulting a third researcher. Results were imported into a PRISMA flowchart. For data collection, relevant data from eligible studies were extracted and crosschecked for accuracy.
Risk of Bias AssessmentThe ROBINS-I tool was used to assess the risk of bias of the included studies.14 For each study, the confounding bias was assessed regarding the selection, measurement interventions, deviations from intended interventions, missing data, outcome assessment, and selection of reported results. The original ROBINS-I tool was adapted to fit the design and specificities of the included studies ().
Data Analysis and Summary of FindingsWhen feasible, a pooled analysis was conducted by applying the Cochran-Mantel-Haenszel method under a random-effects model using Review Manager Software (version 5.3.5). Heterogeneity and sources of variation between studies were assessed through the I2 statistic. Findings were reported according to a narrative synthesis for each outcome of interest and its effect estimate.
RESULTSThe PRISMA flowchart is shown in Figure 1. A total of 4244 references were obtained from MEDLINE and EMBASE searches. After duplicate elimination, 3391 unique references were screened. Of the 3391 unique references, 3312 were excluded based on their title or abstract because they had no relationship with the outcomes of this systematic review. Of these, full texts were obtained for 79 studies. After a detailed assessment of the full texts, 49 studies were excluded. Finally, 27 different studies with published data in 30 publications were included (3 studies published relevant data in 2 separate articles).15–45 The characteristics of the included studies are summarized in .
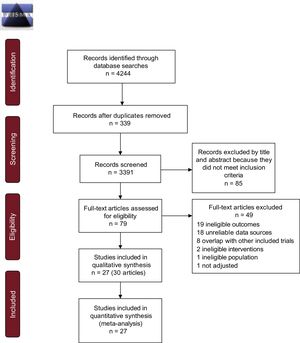
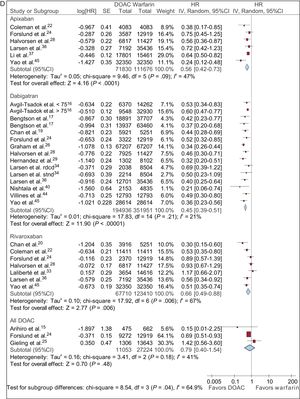
Data from AF patients treated with DOACs vs VKAs up to about 1 year are shown in Figure 2.
HRs with 95%CIs for ischemic stroke (A), ischemic stroke plus systemic embolism (B), major bleeding (C), and intracranial hemorrhage (D) in patients with AF treated with DOACs vs VKAs up to about 1 year. 95%CI, 95% confidence interval; AF, atrial fibrillation; DOACs, direct oral anticoagulants; HR, hazard ratio; IV, interval variable; SE, systemic embolism.
Apixaban and dabigatran did not reduce the risk of ischemic stroke vs warfarin (HR, 0.93; 95% confidence interval [95%CI], 0.71-1.20; HR, 0.95; 95%CI, 0.80-1.13; respectively). Statistical heterogeneity was high among studies. In contrast, rivaroxaban significantly reduced the risk of ischemic stroke vs warfarin (HR, 0.83; 95%CI, 0.73-0.94). This finding was highly consistent among studies (Figure 2A).
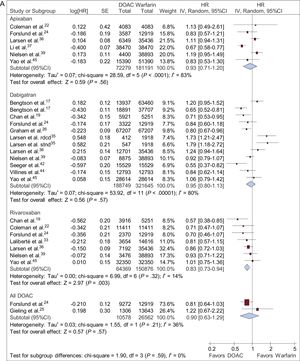
Ischemic Stroke Plus Systemic EmbolismApixaban did not reduce the risk of an ischemic event (stroke/systemic embolism) vs warfarin (HR, 0.88; 95%CI, 0.64-1.21). Statistical heterogeneity was high among studies yielding conflicting results. Dabigatran did not reduce the risk of an ischemic event (stroke/systemic embolism) vs warfarin (HR, 0.92; 95%CI, 0.76-1.11). Rivaroxaban significantly reduced the risk of an ischemic event vs warfarin (HR, 0.80; 95%CI, 0.69-0.93), a finding consistent among studies. Overall, only 1 small study compared DOACs with warfarin with regard to ischemic events, with no significant differences between the 2 groups (HR, 0.44; 95%CI, 0.09-2.04) (Figure 2B).
Major BleedingA risk reduction was observed in major bleeding with apixaban and dabigatran vs warfarin (HR, 0.66; 95%CI, 0.55-0.80; HR, 0.83; 95%CI, 0.70-0.97; respectively), but statistical heterogeneity was high among studies. Rivaroxaban did not show a reduction in the risk of major bleeding vs warfarin (HR, 1.02; 95%CI, 0.95-1.10) (Figure 2C).
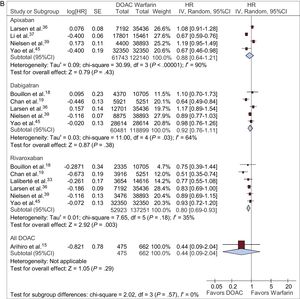
Intracranial HemorrhageApixaban, dabigatran, and rivaroxaban (HR, 0.56; 95%CI, 0.42-0.73; HR, 0.45; 95%CI, 0.39-0.51; HR, 0.66; 95%CI, 0.49-0.88; respectively) significantly reduced the risk of intracranial bleeding vs warfarin (Figure 2D).
Only 4 studies reported results from more than 1 year (Larsen et al.,36 Nielsen et al.,39 Li et al.,37 Avgil-Tsadok et al.16). Larsen et al.36 and Nielsen et al.39 reported data on all of the above outcomes but Li et al.37 and Avgil-Tsadok et al.16 only considered some of them. Compared with studies up to about 1 year of follow-up, the results were unchanged except for apixaban in the outcome “intracranial hemorrhage” and for dabigatran in the outcome “major bleeding”; in these instances, the initially observed significant risk reduction was lost ().
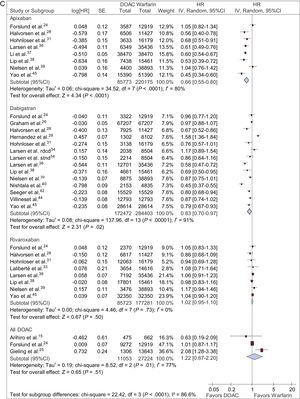
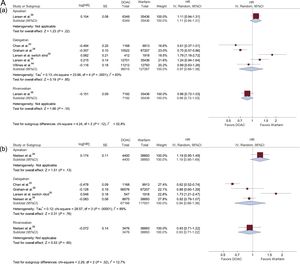
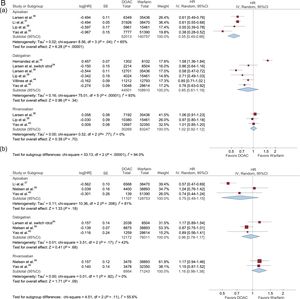
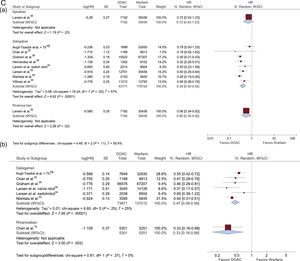
Data on ischemic stroke, major bleeding, and intracranial hemorrhage in patients with AF and treated with DOACs vs VKAs are shown in Figure 3 according to DOAC dose (standard and reduced).
HRs with 95% confidence intervals for ischemic stroke, major bleeding, and intracranial hemorrhage in patients with AF treated with DOACs vs VKAs according to the DOAC dosage (standard/reduced) used in each study. A: ischemic stroke (Aa: Standard dose; Ab: Reduced dose); B: major bleeding (Ba: standard dose; Bb: reduced dose); and C: intracranial bleeding (Ca: standard dose; Cb: reduced dose). 95%CI, 95% confidence interval; AF, atrial fibrillation; DOACs, direct oral anticoagulants; HR, hazard ratio; IV, interval variable; SE, systemic embolism.
Compared with warfarin, treatment with standard doses of apixaban did not reduce the risk of ischemic stroke (HR, 1.11; 95%CI, 0.94-1.31). In addition, treatment with standard doses of dabigatran did not reduce the risk of ischemic stroke (HR, 0.97; 95%CI, 0.68-1.38). However, statistical heterogeneity was high among studies. Treatment with standard doses of rivaroxaban reduced the risk of ischemic stroke vs warfarin (HR, 0.86; 95%CI, 0.72-1.03) (Figure 3A).
Reduced DoseCompared with warfarin, reduced doses of apixaban, dabigatran, or rivaroxaban did not decrease the risk of ischemic stroke (HR, 1.19; 95%CI, 0.95-1.49; HR, 0.94; 95%CI, 0.66-1.36; HR, 0.93; 95%CI, 0.71-1.22; respectively) (Figure 3A).
Major BleedingStandard DoseCompared with warfarin, standard doses of apixaban reduced the risk of major bleeding (HR, 0.55; 95%CI, 0.45-0.66). Treatment with standard doses of dabigatran and rivaroxaban did not reduce the risk of major bleeding (HR, 0.85; 95%CI, 0.61-1.19; HR, 1.02; 95%CI, 0.92-1.12; respectively) (Figure 3B).
Reduced DoseCompared with warfarin, reduced doses of apixaban did not reduce the risk of major bleeding (HR, 0.75; 95%CI, 0.49-1.15). However, statistical heterogeneity was high among studies. Reduced doses of dabigatran did not diminish the risk of major bleeding (HR, 0.96; 95%CI, 0.78-1.17). No risk reduction in major bleeding was observed between reduced doses of rivaroxaban and warfarin (HR, 1.16; 95%CI, 0.98-1.38) (Figure 3B).
Intracranial HemorrhageStandard DoseNo differences in the risk reduction of intracranial bleeding were observed between standard doses of apixaban and warfarin (HR, 0.72; 95%CI, 0.42-1.23). Standard doses of dabigatran (HR, 0.39; 95%CI, 0.30-0.52) and rivaroxaban (HR, 0.56; 95%CI, 0.34-0.92) did reduce the risk of intracranial bleeding over time (Figure 3C).
Reduced DoseNo studies compared the incidences of intracranial bleeding between reduced doses of apixaban and warfarin. Reduced doses of dabigatran (HR, 0.47; 95%CI, 0.39-0.56) and rivaroxaban (HR, 0.33; 95%CI, 0.16-0.68) significantly reduced the risk of intracranial bleeding vs warfarin (Figure 3C).
DISCUSSIONThe results provided by this meta-analysis aid in the understanding of the effectiveness and safety of DOACs in real-life patients. Unlike noncomparative studies, which only include absolute rates,46 this study allows determination of the relative effectiveness and safety of DOACs vs VKAs.
The RE-LY study6 showed that, after a median follow-up of 2.0 years, 150mg dabigatran was associated with a lower risk of stroke or systemic embolism vs warfarin, with a similar risk of major bleeding, whereas 110mg dabigatran was associated with a similar risk of stroke or systemic embolism, but with a lower risk of major bleeding. In the ROCKET-AF trial,7 after a median follow-up of 707 days, rivaroxaban was noninferior to warfarin for the prevention of stroke or systemic embolism, with a similar risk of major bleeding and a lower risk of intracranial and fatal bleeding. In the ARISTOTLE trial, after a median follow-up of 1.8 years, apixaban was superior to warfarin in preventing stroke or systemic embolism, with less bleeding.8 Although these 3 studies were head-to-head with warfarin, the patient populations included were different; thus, no direct comparisons can be made. The characteristics of the included studies are summarized in . As shown in , there were some relevant differences in the clinical characteristics of the patients included in these studies, not only among them, but also among the pivotal clinical trials. As a result, the information provided by this meta-analysis is relevant because it allows determination of the relative effectiveness and safety of DOACs vs VKAs in the full spectrum of real-life patients.
This meta-analysis showed that apixaban and dabigatran did not reduce the risk of ischemic stroke vs warfarin in retrospective claims database studies with a follow-up up to 1 year or with longer-term data. However, rivaroxaban significantly reduced this risk vs warfarin. This significant reduction also occurred with the composite variable of ischemic stroke and systemic embolism. Apixaban and dabigatran significantly reduced major bleeding risk vs warfarin, but not vs rivaroxaban. Notably, statistical heterogeneity was high among studies. However, apixaban, dabigatran, and rivaroxaban significantly reduced the risk of intracranial bleeding vs warfarin. The main objective of anticoagulation in AF patients is to prevent stroke with an acceptable bleeding risk.3 Our data showed that, vs warfarin, the most effective drug to reduce the risk of stroke in clinical practice was rivaroxaban, with a similar risk of major bleeding.
One of the main advantages of DOACs vs warfarin is that DOACs do not require continuous dosage adjustments. DOAC dosages are adjusted according to specific clinical conditions that differ markedly among each drug.6–8 In our meta-analysis, the effectiveness and safety of DOACs was specifically analyzed according to dosage. Standard doses of dabigatran and particularly rivaroxaban, but not apixaban, tended to reduce the risk of ischemic stroke, whereas reduced doses of all DOACs did not decrease the risk of stroke. Reduced doses of DOACs tended to be safer than standard doses in terms of bleeding risk. The main limitation of the observational studies that reported the DOAC doses is that the information regarding the dosage adequacy according to patients’ clinical characteristics was not reported. Our meta-analysis showed that, overall, DOACs at reduced doses were associated with a slightly better safety profile, but with a marked reduction in the effectiveness of stroke prevention. In fact, the prescription of inadequate doses of DOACs is associated with worse safety and no benefit in effectiveness.39,47 This suggests that reduced doses of DOACs should only be used when indicated according to drug labeling and not when physicians perceive an increased risk of bleeding. Unfortunately, inappropriate drug use is reportedly frequent.48–50
A meta-analysis of the effectiveness and safety of DOACs vs VKAs for stroke prevention in AF was recently published.51 However, there are important differences between our systematic review and that published by Ntaios et al.51 Our work included a greater number of studies and a higher number of patients. In addition, the inclusion/exclusion criteria were stricter in our systematic review. Indeed, in our systematic review, studies that exhibited a substantial overlap were excluded. In addition, when data were duplicated, the most recent studies or those with the highest number of patients were selected. Moreover, our search was more up-to-date and we included 11 additional studies vs Ntaios et al.51 Importantly, specific analyses according to follow-up time and DOAC dosage were performed, increasing the validity and generalizability of our results. Accordingly, our systematic review is more complete and exhaustive and has a higher statistical power. For example, due to its higher statistical power and in contrast to the study by Ntaios et al.,51 our work found that dabigatran significantly reduced the risk of major bleeding vs warfarin and that rivaroxaban decreased the risk of ischemic stroke and systemic embolism.
LimitationsSome limitations should be mentioned. Observational studies have a higher risk of bias than clinical trials. We found a moderate risk of bias with the ROBINS-I tool. VKAs were the key comparator arm. However, time in therapeutic range and data adjusted according to this parameter were not disclosed in any studies. Analyses were limited to some outcomes, such as stroke, major bleeding, or intracranial bleeding, but not others. In some comparisons, statistical heterogeneity was high among studies, limiting the validity and the generalizability of the results. No significant clinical information was available for edoxaban.
CONCLUSIONSIn conclusion, in studies with a follow-up up to 1 year or with longer-term data, rivaroxaban significantly reduced the risk of ischemic stroke vs warfarin, unlike apixaban and dabigatran. Apixaban and dabigatran significantly reduced the risk of major bleeding, whereas rivaroxaban showed similar risk to warfarin. Compared with warfarin, DOACs significantly reduced the risk of intracranial bleeding. Reduced doses of DOACs were associated with a marked reduction in the effectiveness of stroke prevention and with a slightly better safety profile. Data from this meta-analysis suggest that, vs warfarin, the effectiveness and safety of some DOACs may differ in real-life AF patients.
FUNDINGBayer Hispania S.L.
CONFLICTS OF INTERESTC. Escobar reports personal fees from Bayer, Boehringer, Bristol-Myers Squibb, Daichii-Sankyo, and Pfizer outside the submitted work. J. Martí-Almor reports personal fees from Bayer, Daiichi-Sankyo, Pfizer, and Boehringer outside the submitted work. A. Pérez Cabeza reports personal fees from Bayer and Daiichi-Sankyo outside the submitted work. M.J. Martínez-Zapata has nothing to disclose.
- -
Data from clinical trials are not always valid for real-life patients.
- -
In recent years, many studies with different designs, analyses, and patient characteristics have analyzed the use of DOACs in AF patients in clinical practice.
- -
This systematic review assessed the effectiveness of DOACs vs VKAs in nonvalvular AF patients.
- -
In contrast to apixaban and dabigatran, rivaroxaban significantly reduced the risk of ischemic stroke vs warfarin.
- -
Apixaban and dabigatran significantly reduced the risk of major bleeding vs warfarin, unlike rivaroxaban, but with a high statistical heterogeneity among studies. All DOACs significantly reduced the risk of intracranial bleeding vs warfarin.
- -
Reduced doses of DOACs were associated with a slightly better safety profile, but with a marked reduction in effectiveness for stroke prevention.
.
Editorial assistance was provided by Content Ed Net, Madrid, Spain.