This study investigated whether the vasoactive inotropic score (VIS) is independently predictive of mortality in cardiogenic shock (CS).
MethodsThis study was retrospective, observational study. Patients who were admitted to the cardiac intensive care unit from January 2012 to December 2015 were screened, and 493 CS patients were finally enrolled. To quantify pharmacologic support, the patients were divided into 5 groups based on a quintile of VIS: 1 to 10, 11 to 20, 21 to 38, 39 to 85, and > 85. The primary outcome was in-hospital mortality.
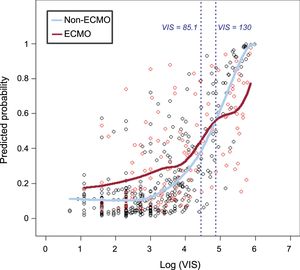
ResultsIn-hospital mortalities in the 5 VIS groups in increasing order were 8.2%, 14.1%, 21.1%, 32.0%, and 65.7%, respectively (P < .001). Multivariable analysis indicated that VIS ranges of 39 to 85 (aOR, 3.85; 95%CI, 1.60-9.22; P = .003) and over 85 (aOR, 10.83; 95%CI, 4.43-26.43; P < .001) remained significant prognostic predictors for in-hospital mortality. With multiple logistic regression to remove any confounding effects, we found that the localized regression lines regarding the odds of death intersected each other's (medical therapy alone and combined extracorporeal membrane oxygenation group) path at VIS = 130. In contrast to linear correlation between VIS and mortality for patients treated with medical therapy alone, there was little association between a VIS of 130 or more and the probability of in-hospital mortality for patients who were treated with extracorporeal membrane oxygenation.
ConclusionsA high level of vasoactive inotropic support during the first 48hours was significantly associated with increased in-hospital mortality in adult CS patients.
Keywords
Cardiogenic shock (CS) is a condition in which cardiac output cannot maintain sufficient end-organ perfusion due to myocardial pump failure, despite normal or elevated preload. Until now, CS has been a life-threatening condition with an overall mortality rate of approximately 40%.1,2 For hemodynamic stabilization, inotropic or vasopressor therapy plays a central role in the initial treatment of patients with CS.3,4 Inotropes or vasopressors can improve hemodynamics in the acute stage through increased myocardial contractility or modification of vascular tone. However, these agents may cause significant adverse events and potential hazards, such as arrhythmia, myocardial ischemia, and peripheral ischemia, which may lead to progression of multiorgan dysfunction syndrome and death.5,6 Several studies showed that the vasoactive inotropic score (VIS), which indicates the amount of cardiovascular support by various inotropes or vasopressors, was independently predictive of clinical outcomes in pediatric patients or adult patients who underwent cardiac surgery.7–9
However, there are limited data on whether the level of pharmacologic cardiovascular support correlates with clinical outcomes in nonsurgical CS patients. Therefore, this study investigated whether the maximum level of pharmacologic cardiovascular support is associated with clinical outcomes in CS patients according to the presence or absence of extracorporeal membrane oxygenation (ECMO).
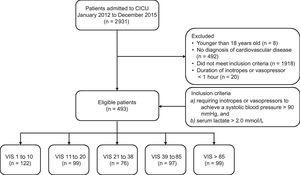
METHODSWe screened 2931 consecutive patients who were admitted to the cardiac intensive care unit (CICU) at Samsung Medical Center from January 2012 to December 2015. Patients were enrolled if they presented with CS regardless of the etiology. We defined CS as follows: a) inotrope or vasopressor support was required to maintain a systolic blood pressure > 90mmHg, and b) accompanying tissue hypoperfusion was represented by serum lactate levels ≥ 2.0 mmol/L. Patients with any of the following criteria were excluded: a) under 18 years old; b) no diagnosis of cardiovascular disease; c) the duration of inotrope or vasopressor use was less than 1 hour, because vasopressor may be used for short duration due to usage of a drug with a vasodilatory effect, such as sedative drugs or vasovagal response without CS in clinical practice. Eligible patients (n = 493) were divided into 5 groups based on the quintile of maximum VIS described by Gaies et al.7 to quantify the level of pharmacologic support for the first 48hours after diagnosis of shock and to investigate the association of the maximum VIS with clinical outcomes in CS patients at variable levels of vasopressors as possible. The VIS groups are 1 to 10, 11 to 20, 21 to 38, 39 to 85, and > 85 (Figure 1). The Institutional Review Board at Samsung Medical Center approved the study protocol and waived the requirement for informed consent.
Scheme of group distribution; 2931 consecutive patients admitted to the CICU between January 2012 and December 2015 were screened. Patients in state a) requiring inotrope or vasopressor support for more than 1 hour to maintain systolic blood pressure > 90mmHg, and b) accompanying tissue hypoperfusion represented by serum lactate level ≥ 2.0 mmol/L due to cardiovascular disease were enrolled. Eligible patients (n = 493) were divided into 5 groups based on quintile of maximum VIS to quantify the level of pharmacologic support for the first 48hours after shock was diagnosed. The 5 groups were VIS of 1 to 10, 11 to 20, 21 to 38, 39 to 85, and > 85. CICU, cardiac intensive care unit; VIS, vasoactive inotropic score.
The VIS was calculated as: dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 10 × milrinone dose (μg/kg/min) + 10 000 × vasopressin dose (unit/kg/min) + 100 × norepinephrine dose (μg/kg/min).7 The hourly doses of dopamine, dobutamine, epinephrine, milrinone, vasopressin, and norepinephrine were retrospectively collected by a trained study coordinator from a review of electronic medical records. Initiation, type, dose-escalation or combination of inotropes or vasopressors, and target blood pressure were left to the individual physician's discretion. The bedside nurses recorded the following information at the moment an event occurred: time of initiation, discontinuation, and dose adjustment of each drug with doses of μg/kg/min, except for vasopressin with a dose of unit/min. The clinical and laboratory data collected on the first day of admission to the CICU were retrospectively obtained, analyzed, and used to calculate Acute Physiology and Chronic Health Evaluation II (APACHE II) scores.
The primary outcome of this study was in-hospital mortality. We also explored CICU mortality, length of stay in the CICU and hospital, the rate of CICU readmission during the same period of hospitalization, and the rate of hospital readmission for acute cardiac care after discharge. Clinical outcomes were recorded in the hospital electronic medical charts. The Korean national database, which employs a citizen registration number that is unique to each individual, was used to determine whether patients died or not.
To compare characteristics and clinical outcomes between the 5 VIS groups, we present continuous variables as mean ± standard deviation or median [interquartile range], and used the analysis of variance or the Kruskal-Wallis test, as appropriate. Categorical variables are described as numbers and percentages and were compared using the chi-square test or the Fisher exact test, when the data were sparse. We also compared groups using the log-rank test for the Kaplan-Meier survival curves. Univariable and multivariable regression analyses were performed to estimate the role of VIS contribution to in-hospital mortality, and to identify risk factors for prediction of in-hospital mortality. Variables that appeared to be related in the univariable analysis with a P-value < .2 were further analyzed in multiple logistic regression models, and a stepwise backward elimination method was used to select predictors of mortality. The odds ratio (OR) of each variable is reported with the 95% confidence interval (95%CI). Assessing the predictive accuracy of the VIS with the area under the curve was carried out using the procedure proposed by DeLong et al.10 We used the robust cross-validated estimation method for the multiple logistic model by leave-one-out cross-validation, and reported its prediction error rate.
We further considered a localized logistic regression model to explore in detail the pattern of mortality as a function of the VIS and the combined use of ECMO support. To accommodate skewness in the VIS, a log transformation was performed before fitting the model. Localized regression lines for ECMO vs non-ECMO groups were then plotted. The intersection at VIS of the 2 lines can be thought of as a turning point, determining when to switch to ECMO to minimize the risk of death.
For all analyses, a 2-tailed test with a P-value < .05 was considered statistically significant. We used SAS 9.2 (SAS Institute Inc, Cary, North Carolina, United States) and R 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria) for Windows for all statistical analyses.
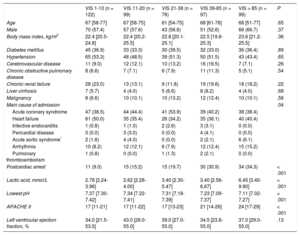
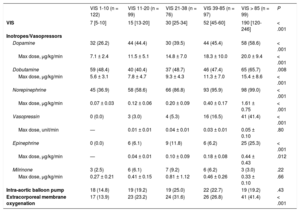
RESULTSThe baseline clinical characteristics of the 493 patients with CS according to VIS are shown in Table 1. Clinical characteristics, including age, sex, and comorbidities were not significantly different among the 5 VIS categories. The median age was 67 (range, 57-77) years and 58.5% of patients were male. With respect to etiologies, acute coronary syndrome and heart failure were responsible for 42.4% and 40.0% of overall CS, respectively. Overall, patients with higher VIS had a higher prevalence of postcardiac arrests, elevated serum lactate levels, and APACHE II scores at CICU admission, whereas arterial pH was significantly lower as VIS increased. The median VIS was 27 (range, 10-60) in patients with CS (Table 2). Except for the VIS 1-10 group, which used dobutamine the most, norepinephrine was the most commonly used cathecholamine (73.0%), and the maximum dose was 0.61 ± 0.74μg/kg/min. Vasopressin was used more frequently as the VIS increased, but the maximum doses were similar. The percentage and the maximum doses of milrinone were not significantly different among the 5 VIS groups. Use of intra-aortic balloon pump among VIS groups was not significantly different (P = .43). However, the use of ECMO (41.4%) was significantly higher in the VIS over 85 group (P < .001).
Baseline Characteristics
| VIS 1-10 (n = 122) | VIS 11-20 (n = 99) | VIS 21-38 (n = 76) | VIS 39-85 (n = 97) | VIS > 85 (n = 99) | P | |
|---|---|---|---|---|---|---|
| Age | 67 [58-77] | 67 [58-75] | 61 [54-75] | 68 [61-78] | 68 [51-77] | .65 |
| Male | 70 (57.4) | 57 (57.6) | 43 (56.6) | 51 (52.6) | 66 (66.7) | .37 |
| Body mass index, kg/m2 | 22.4 [20.5-24.8] | 22.4 [20.2-25.5] | 22.8 [20.1-25.1] | 22.5 [19.8-25.3] | 23.6 [21.2-25.5] | .36 |
| Diabetes mellitus | 45 (36.9) | 33 (33.3) | 30 (39.5) | 32 (33.0) | 36 (36.4) | .89 |
| Hypertension | 65 (53.3) | 48 (48.5) | 39 (51.3) | 50 (51.5) | 43 (43.4) | .65 |
| Cerebrovascular disease | 11 (9.0) | 12 (12.1) | 10 (13.2) | 16 (16.5) | 7 (7.1) | .26 |
| Chronic obstructive pulmonary disease | 8 (6.6) | 7 (7.1) | 6 (7.9) | 11 (11.3) | 5 (5.1) | .54 |
| Chronic renal failure | 28 (23.0) | 13 (13.1) | 9 (11.8) | 19 (19.6) | 18 (18.2) | .22 |
| Liver cirrhosis | 7 (5.7) | 4 (4.0) | 5 (6.6) | 8 (8.2) | 4 (4.0) | .68 |
| Malignancy | 8 (6.6) | 10 (10.1) | 10 (13.2) | 12 (12.4) | 10 (10.1) | .56 |
| Main cause of admission | .04 | |||||
| Acute coronary syndrome | 47 (38.5) | 44 (44.4) | 41 (53.9) | 39 (40.2) | 38 (38.4) | |
| Heart failure | 61 (50.0) | 35 (35.4) | 26 (34.2) | 35 (36.1) | 40 (40.4) | |
| Infective endocarditis | 1 (0.8) | 1 (1.0) | 2 (2.6) | 3 (3.1) | 0 (0.0) | |
| Pericardial disease | 0 (0.0) | 3 (3.0) | 0 (0.0) | 4 (4.1) | 0 (0.0) | |
| Acute aortic syndrome* | 2 (1.6) | 4 (4.0) | 0 (0.0) | 2 (2.1) | 6 (6.1) | |
| Arrhythmia | 10 (8.2) | 12 (12.1) | 6 (7.9) | 12 (12.4) | 15 (15.2) | |
| Pulmonary thromboembolism | 1 (0.8) | 0 (0.0) | 1 (1.3) | 2 (2.1) | 0 (0.0) | |
| Postcardiac arrest | 11 (9.0) | 15 (15.2) | 15 (19.7) | 30 (30.9) | 34 (34.3) | < .001 |
| Lactic acid, mmol/L | 2.78 [2.24-3.96] | 2.62 [2.28-4.00] | 3.40 [2.30-5.47] | 3.40 [2.56-6.67] | 6.45 [3.40-9.90] | < .001 |
| Lowest pH | 7.37 [7.30-7.42] | 7.34 [7.22-7.41] | 7.31 [7.18-7.39] | 7.23 [7.09-7.37] | 7.11 [7.02-7.27] | < .001 |
| APACHE II | 17 [11-21] | 17 [11-22] | 17 [13-23] | 21 [14-26] | 24 [17-29] | < .001 |
| Left ventricular ejection fraction, % | 34.0 [21.5-53.5] | 43.0 [28.0-55.0] | 39.0 [27.0-55.0] | 34.5 [23.8-55.0] | 37.0 [29.0-55.0] | .13 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; VIS, vasoactive inotrope score.
Values are expressed as median [interquartile range] or No. (%)
Treatment Characteristics
| VIS 1-10 (n = 122) | VIS 11-20 (n = 99) | VIS 21-38 (n = 76) | VIS 39-85 (n = 97) | VIS > 85 (n = 99) | P | |
|---|---|---|---|---|---|---|
| VIS | 7 [5-10] | 15 [13-20] | 30 [25-34] | 52 [45-60] | 190 [120-246] | < .001 |
| Inotropes/Vasopressors | ||||||
| Dopamine | 32 (26.2) | 44 (44.4) | 30 (39.5) | 44 (45.4) | 58 (58.6) | < .001 |
| Max dose, μg/kg/min | 7.1 ± 2.4 | 11.5 ± 5.1 | 14.8 ± 7.0 | 18.3 ± 10.0 | 20.0 ± 9.4 | < .001 |
| Dobutamine | 59 (48.4) | 40 (40.4) | 37 (48.7) | 46 (47.4) | 65 (65.7) | .008 |
| Max dose, μg/kg/min | 5.6 ± 3.1 | 7.8 ± 4.7 | 9.3 ± 4.3 | 11.3 ± 7.0 | 15.4 ± 8.6 | < .001 |
| Norepinephrine | 45 (36.9) | 58 (58.6) | 66 (86.8) | 93 (95.9) | 98 (99.0) | < .001 |
| Max dose, μg/kg/min | 0.07 ± 0.03 | 0.12 ± 0.06 | 0.20 ± 0.09 | 0.40 ± 0.17 | 1.61 ± 0.75 | < .001 |
| Vasopressin | 0 (0.0) | 3 (3.0) | 4 (5.3) | 16 (16.5) | 41 (41.4) | < .001 |
| Max dose, unit/min | — | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.10 | .80 |
| Epinephrine | 0 (0.0) | 6 (6.1) | 9 (11.8) | 6 (6.2) | 25 (25.3) | < .001 |
| Max dose, μg/kg/min | — | 0.04 ± 0.01 | 0.10 ± 0.09 | 0.18 ± 0.08 | 0.44 ± 0.43 | .012 |
| Milrinone | 3 (2.5) | 6 (6.1) | 7 (9.2) | 6 (6.2) | 3 (3.0) | .22 |
| Max dose, μg/kg/min | 0.27 ± 0.21 | 0.41 ± 0.15 | 0.81 ± 1.12 | 0.46 ± 0.26 | 0.33 ± 0.10 | .66 |
| Intra-aortic balloon pump | 18 (14.8) | 19 (19.2) | 19 (25.0) | 22 (22.7) | 19 (19.2) | .43 |
| Extracorporeal membrane oxygenation | 17 (13.9) | 23 (23.2) | 24 (31.6) | 26 (26.8) | 41 (41.4) | < .001 |
VIS, vasoactive inotrope score.
Values are expressed as mean ± standard deviation and median [interquartile range] or No. (%).
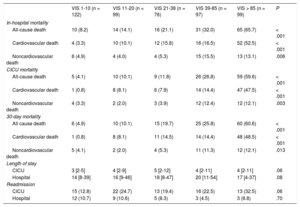
Overall, 136 (27.6%) patients died while hospitalized, and 109 (22.1%) of them died in the CICU (Table 3). In-hospital mortality rates were higher in patients with higher VIS (8.2%, 14.1%, 21.1%, 32.0%, 65.7% in order of VIS < 10, 11-20, 21-38, 39-85, and VIS > 85, respectively with P < .001). Noncardiovascular deaths comprised nearly one-third of all deaths, and these, as well as cardiovascular deaths, increased as the VIS increased.
Clinical Outcomes
| VIS 1-10 (n = 122) | VIS 11-20 (n = 99) | VIS 21-38 (n = 76) | VIS 39-85 (n = 97) | VIS > 85 (n = 99) | P | |
|---|---|---|---|---|---|---|
| In-hospital mortality | ||||||
| All-cause death | 10 (8.2) | 14 (14.1) | 16 (21.1) | 31 (32.0) | 65 (65.7) | < .001 |
| Cardiovascular death | 4 (3.3) | 10 (10.1) | 12 (15.8) | 16 (16.5) | 52 (52.5) | < .001 |
| Noncardiovascular death | 6 (4.9) | 4 (4.0) | 4 (5.3) | 15 (15.5) | 13 (13.1) | .006 |
| CICU mortality | ||||||
| All-cause death | 5 (4.1) | 10 (10.1) | 9 (11.8) | 26 (26.8) | 59 (59.6) | < .001 |
| Cardiovascular death | 1 (0.8) | 8 (8.1) | 6 (7.9) | 14 (14.4) | 47 (47.5) | < .001 |
| Noncardiovascular death | 4 (3.3) | 2 (2.0) | 3 (3.9) | 12 (12.4) | 12 (12.1) | .003 |
| 30-day mortality | ||||||
| All cause death | 6 (4.9) | 10 (10.1) | 15 (19.7) | 25 (25.8) | 60 (60.6) | < .001 |
| Cardiovascular death | 1 (0.8) | 8 (8.1) | 11 (14.5) | 14 (14.4) | 48 (48.5) | < .001 |
| Noncardiovascular death | 5 (4.1) | 2 (2.0) | 4 (5.3) | 11 (11.3) | 12 (12.1) | .013 |
| Length of stay | ||||||
| CICU | 3 [2-5] | 4 [2-9] | 5 [2-12] | 4 [2-11] | 4 [2-11] | .06 |
| Hospital | 14 [8-39] | 16 [9-46] | 18 [8-47] | 20 [11-54] | 17 [4-37] | .08 |
| Readmission | ||||||
| CICU | 15 (12.8) | 22 (24.7) | 13 (19.4) | 16 (22.5) | 13 (32.5) | .06 |
| Hospital | 12 (10.7) | 9 (10.6) | 5 (8.3) | 3 (4.5) | 3 (8.8) | .70 |
CICU, cardiac intensive care unit; VIS, vasoactive inotrope score.
Values are median [interquartile range] or No. (%).
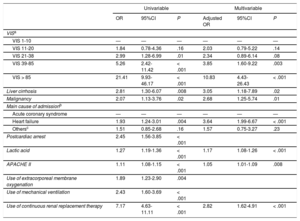
Clinical variables that were considered as possible prognostic factors on the univariable logistic regression analysis included liver cirrhosis, malignancy, primary cardiovascular problems, a history of cardiac arrest, lactic acid level, APACHE II, use of ECMO, mechanical ventilation, and continuous renal replacement therapy. After adjustment of the model for these covariates, the adjusted OR of VIS groups increased from 2.03 (95%CI, 0.79-5.22; P = .14), 2.34 (95%CI, 0.89-6.14; P = .08), 3.85 (95%CI, 1.60-9.22; P = .003) to 10.83 (95%CI, 4.43-26.43; P < .001) for VIS ranges of 11-20, 21-38, 39-85, and > 85, respectively, compared with the VIS of 1 to 10 (Table 4). Vasoactive inotropic score remained significant prognostic predictors for in-hospital mortality, especially those over 39. Among our patients, 52.9% and 29.8% of them used mechanical ventilation and continuous renal replacement therapy for a median 4 (2-8) days and 4 (3-8) days, respectively. The proportion of patients using mechanical ventilation and continuous renal replacement therapy increased as the VIS increased, but the durations of mechanical ventilation and continuous renal replacement therapy were similar among the 5 VIS groups. Overall, the multivariable prediction model had an area under the curve of 0.8676 (95%CI, 0.83-0.90) and the goodness-of-fit of the model was also verified using the Osius-Rojek test (P-value = .835). Our model resulted in a misclassification error rate of 16.4% via leave-one-out cross-validatioin. Neither length of stay nor readmission rates differed among the 5 VIS groups.
Predictors of In-hospital Mortality
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | Adjusted OR | 95%CI | P | |
| VISa | ||||||
| VIS 1-10 | — | — | — | — | — | — |
| VIS 11-20 | 1.84 | 0.78-4.36 | .16 | 2.03 | 0.79-5.22 | .14 |
| VIS 21-38 | 2.99 | 1.28-6.99 | .01 | 2.34 | 0.89-6.14 | .08 |
| VIS 39-85 | 5.26 | 2.42-11.42 | < .001 | 3.85 | 1.60-9.22 | .003 |
| VIS > 85 | 21.41 | 9.93-46.17 | < .001 | 10.83 | 4.43-26.43 | < .001 |
| Liver cirrhosis | 2.81 | 1.30-6.07 | .008 | 3.05 | 1.18-7.89 | .02 |
| Malignancy | 2.07 | 1.13-3.76 | .02 | 2.68 | 1.25-5.74 | .01 |
| Main cause of admissionb | ||||||
| Acute coronary syndrome | — | — | — | — | — | — |
| Heart failure | 1.93 | 1.24-3.01 | .004 | 3.64 | 1.99-6.67 | < .001 |
| Othersc | 1.51 | 0.85-2.68 | .16 | 1.57 | 0.75-3.27 | .23 |
| Postcardiac arrest | 2.45 | 1.56-3.85 | < .001 | |||
| Lactic acid | 1.27 | 1.19-1.36 | < .001 | 1.17 | 1.08-1.26 | < .001 |
| APACHE II | 1.11 | 1.08-1.15 | < .001 | 1.05 | 1.01-1.09 | .008 |
| Use of extracorporeal membrane oxygenation | 1.89 | 1.23-2.90 | .004 | |||
| Use of mechanical ventilation | 2.43 | 1.60-3.69 | < .001 | |||
| Use of continuous renal replacement therapy | 7.17 | 4.63-11.11 | < .001 | 2.82 | 1.62-4.91 | < .001 |
95%CI, 95%confidence interval; APACHE II, Acute Physiology and Chronic Health Evaluation II; OR, odds ratio; VIS, vasoactive inotropes score.
As a further close look at the in-hospital mortality, we explored the role of VIS and ECMO. In crude analysis, in-hospital death was higher within VIS 1-85, and it was lower in VIS over 85 in the ECMO group compared with the non-ECMO group, and this interaction was highly significant (P = .007). We considered a localized logistic regression model to predict mortality, aiming to find a pattern of predicted mortality that was associated with VIS according to the combined use of ECMO support. We found that the localized regression lines intersected each other's path at VIS = 130 (Figure 2). However, they started out quite differently and, from about VIS = 85, they converged to VIS = 130, at which point they crossed each other's path. This suggests that VIS 85 to 130 is the turning point at which the odds of death in patients receiving pharmacologic support alone are worse than the odds of death in patients receiving combined ECMO support.
Predicted probability of in-hospital mortality vs log (VIS). Predicted probability of in-hospital mortality in patients receiving pharmacologic support alone (non-ECMO) was significantly lower than in patients who underwent ECMO within VIS less than 85. However the difference between 2 groups began to decrease from about VIS = 85 and the predicted probability of non-ECMO group was significantly higher than that of the ECMO group with VIS above 130. ECMO, extracorporeal membrane oxygenation; VIS, vasoactive inotropic score.
The present study investigated the association between the maximum level of pharmacologic cardiovascular support for the first 48hours after shock and clinical outcomes in nonsurgical adult patients with CS. The major findings of the study are as follows: a) VIS reflecting the amount of inotropic or vasopressor therapy is associated with in-hospital and CICU mortalities in patients with CS who require cardiac critical care; b) VIS was a significant prognostic factor for in-hospital mortality in the multivariable logistic regression model; c) VIS has a higher predictive performance for in-hospital and CICU mortalities than APACHE II and serum lactate level; and d) in VIS of 130 or more, in contrast to patients treated with medical therapy alone, patients treated with ECMO showed a weak correlation between VIS and predicted probability of in-hospital mortality.
The present study is in agreement with previous studies that demonstrated an association between high VIS and poor clinical outcomes after cardiac surgery in pediatric and adult patients. In 2010, Gaies et al.7 introduced the concept of VIS in a study of infants after cardiopulmonary bypass and found that high VIS patients (defined as VIS of 20 or over in the first 24hours or VIS of 15 or over within the next 24hours) had a significantly higher risk for composite endpoints of death, mechanical circulatory support, renal replacement therapy, cardiac arrest and central nervous system injury. Since then, several studies have demonstrated the capability of VIS as a predictor of morbidity and mortality in child and adolescent patients following cardiac surgery.11,12 Although Geppert et al.13 did not use the VIS in a study of patients with CS with complicating acute myocardial infarction, they found 30-day mortality rates of 86% and 100% when the sum of epinephrine and norepinephrine doses was higher than 0.31μg/kg/min and 1.16μg/kg/min, respectively.
Because of its effect on the cardiovascular system, inotropic and vasopressor therapy is an essential part of the treatment of CS. However, this therapy can also be related to adverse cardiovascular events such as hypertension/hypotension, arrhythmias, peripheral and cardiac ischemia, which may be fatal.5 Catecholamine usage can progress to multiple organ dysfunction and ultimately make it more difficult to recover from CS because of catecholamine-induced metabolic changes, including increased oxygen consumption, increased glycolysis, glycogenolysis and lipolysis, increased gluconeogenesis and ketogenesis, increased peripheral insulin resistance, and increased lactate release.14 Furthermore, acidosis may decrease the effect of inotropic or vasopressor drugs and may frequently be a reason for increasing doses. The present study showed an increase in noncardiovascular deaths, as well as cardiovascular deaths, in patients with higher VIS. Sepsis accounted for 69.0% of noncardiovascular deaths, and sepsis-related deaths were higher in high VIS patients (P = .001), although there was no difference between VIS 39 to 85 and VIS over 85. A study that examined the perioperative VIS in patients undergoing heart transplantation also showed that patients with VIS of 20 and over had a significantly higher incidence of infection.9 Catecholamines are known to be related to immunosuppression, stimulation of bacterial proliferation and biofilm formation, and they lead to increased infection risk.15–17
Interestingly, this study showed a different pattern of predicted in-hospital mortality according to VIS between patients who received pharmacologic support alone and patients who received combined ECMO support. Although the predicted in-hospital mortality in the ECMO group was significantly higher than in the non-ECMO group within VIS less than 85, the odds of death in the ECMO group were similar to those of the non-ECMO group within the range from 85 to 130. The predicted in-hospital mortality of the ECMO group was lower than that in the non-ECMO group with VIS of 130 and over. Certain potential benefits of ECMO support are derived from its capability to reverse the metabolic derangement and deleterious systemic effects of CS.18,19 Consequently, both American and European guidelines for the management of ST-elevation myocardial infarction suggest that left ventricular assist devices be considered for circulatory support in patients with refractory CS.20,21 Samuels et al.22 showed that the number and level of inotropic supports correlates with hospital mortality in postcardiotomy CS patients. In particular, the hospital mortality for patients requiring 2 or more high dose inotropes was more than 40%, and the authors considered that dosage requirement as a pharmacological criterion for ventricular assist device insertion in postcardiotomy shock. Converting to VIS, this criterion corresponds to VIS > 15-27 in a 60 kilogram person, which is a relatively low score compared with our study. This discrepancy might be partially related to differences in the enrolled patients’ characteristics between the 2 studies. This study did not include patients in the immediate postoperative period. Moreover, the predicted mortality in patients was directly compared according to whether they were using ECMO or not, in order to weigh the benefit of ECMO against the risk involved. In contrast to the positive effect of ECMO with VIS > 130, the higher predicted mortality of the ECMO group with VIS < 85 might be explained by an ECMO-related systemic inflammatory response syndrome, as well as other ECMO complications such as bleeding, infection, and limb ischemia.23–26 These findings suggest that initiation of mechanical circulatory support like ECMO should be considered in selected CS patients with VIS over 85, despite the potential risks and complications associated with circulatory assist devices.
LimitationsThe present study may have several limitations. First, because our study was conducted retrospectively, there were no predefined criteria about initial and combination regimens, dose adjustments of vasoactive drugs and target blood pressure, as well as timing of ECMO implementation. Further randomized controlled studies employing established protocols for vasoactive drug and ECMO usage are needed to elucidate the role of VIS and ECMO in CS. Second, hemodynamic criteria such as a cardiac index, left ventricular or right ventricular end-diastolic pressure were not included in defining shock. Therefore, we could not exclude a postcardiac arrest syndrome, where a distributive shock component predominates, particularly in postcardiac arrest patients. Third, we used VIS as a way to quantify the level of vasopressor or inotropic support and to reflect the equivalence of the hemodynamic support property of each drug. However, the maximum VIS has inherent limitations in assessing the patient's hemodynamic status because the maximum dose of each vasopressor was influenced by various medical conditions such as sedative drugs, application of mechanical ventilation, and body surface area. Also, this study only included drugs in the VIS formula. Fourth, we assumed that the intra-aortic balloon pump has little effect on clinical outcomes. This assumption was based on previous studies that demonstrated no positive effect of intra-aortic balloon pump on mortality in patients with acute myocardial infarction complicated by CS.3,27,28 Last, we used the APACHE II score to assess the capability of VIS as a predictor of mortality because of the absence of a gold standard for a risk-scoring system in CS. Although the APACHE II score was validated in a previous study that included patients with various cardiovascular diseases, our predictive model may be inadequate to reflect the unique characteristics of patients with CS.29
CONCLUSIONSA high VIS was independently associated with increased in-hospital and CICU mortalities, although there was little association between VIS and the probability of in-hospital mortality for patients who were treated with ECMO and very high levels of vasopressors, and had a high predictive performance for in-hospital and CICU mortalities in patients with CS requiring cardiac critical care. These findings suggest that the decision to increase vasopressor or inotrope dosage should be carefully decided after considering a risk-benefit analysis of the drugs.
CONFLICTS OF INTERESTNone declared.
- -
Inotropes or vasopressors can improve hemodynamics in the acute stage through increased myocardial contractility or modification of vascular tone. However, these agents may cause significant adverse events and potential hazards, which may lead to progression of multiorgan dysfunction syndrome and death. In addition, VIS reflecting the amount of inotropic or vasopressor therapy was independently predictive of clinical outcomes in pediatric patients or adult patients who underwent cardiac surgery.
- -
Vasoactive inotropic score is associated with in-hospital and CICU mortalities in patients with CS who require cardiac critical care and has a higher predictive performance for in-hospital and CICU mortalities than APACHE II and serum lactate level. Particularly in VIS of 130 or more, unlike patients treated with medical therapy alone, patients treated with ECMO had a weak correlation between VIS and predicted probability of in-hospital mortality.