Acute kidney injury (AKI) is frequently observed after transcatheter aortic valve implantation (TAVI) and is associated with higher mortality. However, the impact of AKI on long-term outcomes remains controversial. Therefore, we sought to evaluate the impact of AKI on short- and long-term outcomes following TAVI using the Valve Academic Research Consortium 2 criteria.
MethodsConsecutive patients (n = 794) with severe aortic stenosis who underwent TAVI were included in a multicenter Brazilian registry. Logistic regression analysis was used to identify predictors of AKI. Four-year outcomes were determined as Kaplan-Meier survival curves, and an adjusted landmark analysis was used to test the impact of AKI on mortality among survivors at 12 months.
ResultsThe incidence of AKI after TAVI was 18%. Independent predictors of AKI were age, diabetes mellitus, major or life-threatening bleeding and valve malpositioning. Acute kidney injury was independently associated with higher risk of all-cause death (adjusted HR, 2.8; 95%CI, 2.0-3.9; P < .001) and cardiovascular mortality (adjusted HR, 2.9; 95%CI, 1.9-4.4; P < .001) over the entire follow-up period. However, when considering only survivors at 12 months, there was no difference in both clinical endpoints (adjusted HR, 1.2; 95%CI, 0.5-2.4; P = .71, and HR, 0.7; 95%CI, 0.2-2.1; P = .57, respectively).
ConclusionsAcute kidney injury is a frequent complication after TAVI. Older age, diabetes, major or life-threatening bleeding, and valve malpositioning were independent predictors of AKI. Acute kidney injury is associated with worse short- and long-term outcomes. However, the major impact of AKI on mortality is limited to the first year after TAVI.
Keywords
Transcatheter aortic valve implantation (TAVI) is now a well-established treatment for inoperable, high and intermidiate surgical risk patients with symptomatic severe aortic stenosis.1–7 Acute kidney injury (AKI) is frequently observed after TAVI, with rates ranging from 3% to 50% depending on the definition used.8–16
The occurrence of AKI following TAVI has been associated with the presence of comorbidities, the administration of contrast medium, the need for rapid pacing with subsequent hypotension,17 and the occurrence of postprocedural complications, such as bleeding and vascular complications.13
Acute kidney injury following TAVI is associated with poorer short- and mid-term outcomes,9 prolonged hospital stay and, consequently, increased financial health care system cost.18,19 However, conflicting evidence is available regarding the impact of AKI on long-term clinical outcomes.10,13 Additionally, previous studies have used heterogeneous definitions of AKI, and have only assessed short- and mid-term clinical outcomes.9,10 Moreover, the vast majority of previous research dichotomized AKI as present or absent and did not stratify this complication according to its different stages, even though a few studies have shown that the risk of death and complications increases with higher AKI severity.19,20 Therefore, our study aimed to evaluate the clinical impact of AKI and its different stages on short- and long-term clinical outcomes following TAVI using the Valve Academic Research Consortium 2 (VARC-2) criteria.21 We also aimed to identify the independent predictors of AKI after TAVI, in an attempt to better define risk assessment for this increasing population.
METHODSStudy PopulationFrom January 2008 to January 2015, 819 consecutive patients with symptomatic severe aortic stenosis underwent TAVI and were included in a multicenter Brazilian registry.22 Twenty-two sites from different regions of Brazil participated in the study. For each patient, baseline characteristics, procedure details, and follow-up data were collected and recorded in a web-based case report form designed especially for this registry. To better understand the predictors and prognostic role of AKI following TAVI, we excluded 25 patients who died in the first 24hours after the procedure. Finally, 794 participants were included in this analysis. Informed written consent was obtained from the patients included prospectively. The study protocol was conducted in accordance with the Declaration of Helsinki and was approved by each institution's ethics committee.
ProcedureThe self-expandable CoreValve (Medtronic, Minneapolis, Minnesota, United States), the balloon-expandable SAPIEN XT (Edwards Lifesciences, Irvine, California, United States) or the Inovare (Braile Biomédica, São José do Rio Preto, São Paulo, Brazil) prosthesis were used. Transcatheter aortic valve implantation procedures were performed according to standard techniques. The type of anesthesia used and access site were left to the operator's discretion. Transfemoral vascular access was the first-choice approach. When transfemoral access was not feasible, transapical or transarterial approaches (transubclavian, direct transaortic, transapical or transcarotid) were used according to the preference of the Heart Team.
Clinical Endpoints and DefinitionsPatients were followed up and all adverse events were adjudicated by an independent committee, according to the VARC-2 definitions. The definition of AKI was based on the AKIN (Acute Kidney Injury Network) criteria as follows: stage 1 (increase in serum creatinine to 150%-199% compared with baseline or increase of 0.3mg/dL or urine output < 0.5mL/kg/h for > 6 but < 12h); stage 2 (increase in serum creatinine to 200%-299% compared with baseline or urine output < 0.5mL/kg/h for > 12 but < 24h); stage 3 (increase in serum creatinine to > 300% compared with baseline or serum creatinine of > 4.0mg/dL with an acute increase of at least 0.5mg/dL or urine output < 0.3mL/kg/h for > 24h or anuria for > 12h or renal replacement therapy). The baseline serum creatinine was measured at the hospital admission before the procedure and daily thereafter, up to 1 week or less if the patient was discharged before this period. In comparison with the original VARC definitions, the timing for the diagnosis of AKI was extended from 72hours to 7 days. Chronic kidney disease was defined as estimated glomerular filtration rate < 60mL/min. The definition of valve malpositioning included valve migration, valve embolization, and ectopic valve deployment.21 Device success was defined as the absence of procedural mortality and correct positioning of a single prosthetic heart valve into the proper anatomical location and intended performance of the prosthetic heart valve (mean aortic valve gradient < 20mmHg and no moderate or severe aortic valve regurgitation).21 The present study reports data on clinical outcomes at 30 days, 1 year, over the entire length of follow-up (up to 4 years), and starting at 1 year of follow-up (landmark analysis).
Statistical AnalysisThe baseline clinical and procedural characteristics of the study population are presented according to AKI status. Continuous variables are reported as mean ± standard deviation, or median and [interquartile range], and were compared using the Student t test or the Mann-Whitney test, as appropriate. Categorical variables are reported as frequencies and percentages, and were compared using the Pearson chi-square test.
Kaplan-Meier survival curves were created using different stages of AKI as a cutoff and the outcomes were compared using a log-rank test. Once AKI was associated with multiple factors including patient comorbidities, the procedure itself and its complications, a landmark survival analysis was used to explore the clinical impact of AKI on short- and long-term outcomes starting from 30 days and 1 year after the index procedure, respectively. Cox proportional hazards models were used to test the impact of AKI on all-cause death and cardiovascular mortality. Variables were selected if P < .2 in a bivariate analysis, and all variables with P < .05 remained in the model. The variables included in the models were age, sex, New York Heart Association functional class, chronic obstructive pulmonary disease, peripheral vascular disease, aortic balloon valvuloplasty, left ventricular ejection fraction, moderate or severe mitral regurgitation, pulmonary hypertension, access site (femoral vs nonfemoral), transoesophageal echocardiogram, predilatation, AKI, myocardial infarction, stroke, major or life-threatening bleeding, major vascular complication, and valve malpositioning.
A stepwise logistic regression analysis including all variables with P value < .2 in the univariate analysis was used to identify the predictors of AKI and 30-day all-cause and cardiovascular mortality. The variables tested in the models were age, chronic kidney disease, body mass index, diabetes mellitus, peripheral vascular disease, systemic hypertension, porcelain aorta, diuretics use, contrast media volume, creatinine clearance, valve malpositioning, major or life-threatening bleeding, and major vascular complications. All statistical tests were 2-sided, and the criterion for statistical significance was P < .05. All statistical analyses were performed using SPSS statistical software version 20.0.
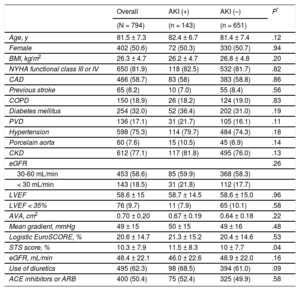
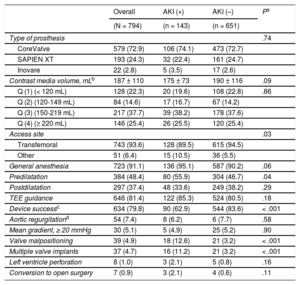
RESULTSBaseline Clinical CharacteristicsThe baseline characteristics of the study population are illustrated in Table 1. The overall mean age was 81.5 ± 7.3 years, 50.6% were women, 32.0% diabetics, the mean Society of Thoracic Surgeons score was 10.3 ± 7.9, and the mean EuroSCORE was 20.6 ± 14.7. The mean left ventricular ejection fraction was 58.6 ± 15% and the mean aortic gradient was 49 ± 15mmHg. The preprocedural estimated glomerular filtration rate was 48.4 ± 22.1mL/min and 77.1% of the patients had chronic kidney disease.
Baseline Clinical Characteristics of the Study Population According to AKI Status
| Overall | AKI (+) | AKI (–) | P* | |
|---|---|---|---|---|
| (N = 794) | (n = 143) | (n = 651) | ||
| Age, y | 81.5 ± 7.3 | 82.4 ± 6.7 | 81.4 ± 7.4 | .12 |
| Female | 402 (50.6) | 72 (50.3) | 330 (50.7) | .94 |
| BMI, kg/m2 | 26.3 ± 4.7 | 26.2 ± 4.7 | 26.8 ± 4.8 | .20 |
| NYHA functional class III or IV | 650 (81.9) | 118 (82.5) | 532 (81.7) | .82 |
| CAD | 466 (58.7) | 83 (58) | 383 (58.8) | .86 |
| Previous stroke | 65 (8.2) | 10 (7.0) | 55 (8.4) | .56 |
| COPD | 150 (18.9) | 26 (18.2) | 124 (19.0) | .83 |
| Diabetes mellitus | 254 (32.0) | 52 (36.4) | 202 (31.0) | .19 |
| PVD | 136 (17.1) | 31 (21.7) | 105 (16.1) | .11 |
| Hypertension | 598 (75.3) | 114 (79.7) | 484 (74.3) | .18 |
| Porcelain aorta | 60 (7.6) | 15 (10.5) | 45 (6.9) | .14 |
| CKD | 612 (77.1) | 117 (81.8) | 495 (76.0) | .13 |
| eGFR | .26 | |||
| 30-60 mL/min | 453 (58.6) | 85 (59.9) | 368 (58.3) | |
| < 30 mL/min | 143 (18.5) | 31 (21.8) | 112 (17.7) | |
| LVEF | 58.6 ± 15 | 58.7 ± 14.5 | 58.6 ± 15.0 | .96 |
| LVEF < 35% | 76 (9.7) | 11 (7.9) | 65 (10.1) | .58 |
| AVA, cm2 | 0.70 ± 0.20 | 0.67 ± 0.19 | 0.64 ± 0.18 | .22 |
| Mean gradient, mmHg | 49 ± 15 | 50 ± 15 | 49 ± 16 | .48 |
| Logistic EuroSCORE, % | 20.6 ± 14.7 | 21.3 ± 15.2 | 20.4 ± 14.6 | .53 |
| STS score, % | 10.3 ± 7.9 | 11.5 ± 8.3 | 10 ± 7.7 | .04 |
| eGFR, mL/min | 48.4 ± 22.1 | 46.0 ± 22.6 | 48.9 ± 22.0 | .16 |
| Use of diuretics | 495 (62.3) | 98 (68.5) | 394 (61.0) | .09 |
| ACE inhibitors or ARB | 400 (50.4) | 75 (52.4) | 325 (49.9) | .58 |
ACE, angiotensin-converting enzyme; AKI, acute kidney injury; ARB, angiotensin receptor blocker; AVA, aortic valve area; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PVD, peripheral vascular disease; STS, Society of Thoracic Surgeons.
Data are expressed as No. (%) or mean ± standard deviation.
The incidence of AKI following TAVI was 18% (143/794). Of these, 11.1% (88) were classified as AKI stage 1, 2.4% (19) as stage 2, and 4.5% (36) as stage 3. Demographic characteristics were similar between patients with and without AKI (Table 1). The only exception was the Society of Thoracic Surgeons score, which was higher among patients with AKI (11.5 ± 8.3 vs 10 ± 7.7; P = .04). Length of hospital stay was significantly higher among patients with AKI (19.3 ± 29.1 days vs 11.2 ± 20.9 days; P < .001).
Procedural Characteristics and ComplicationsTranscatheter aortic valve implantation was performed via transfemoral access in most patients (93.6%), with CoreValve being the device most commonly used (72.9%). Most patients underwent general anesthesia. Procedural characteristics are shown in Table 2. We observed a higher prevalence of nontransfemoral access and predilatation among patients who developed AKI. The mean total contrast media used was 187 ± 110mL, with a nonsignificant trend toward less contrast use in the group who developed AKI (175 ± 73 vs 190 ± 116; P = .09). However, the amount of contrast media received was higher among patients with valve malpositioning (276 ± 219mL vs 183 ± 100mL; P = .028) in comparison with those without valve malpositioning.
Procedural Characteristics and Complications According to AKI Status
| Overall | AKI (+) | AKI (–) | Pa | |
|---|---|---|---|---|
| (N = 794) | (n = 143) | (n = 651) | ||
| Type of prosthesis | .74 | |||
| CoreValve | 579 (72.9) | 106 (74.1) | 473 (72.7) | |
| SAPIEN XT | 193 (24.3) | 32 (22.4) | 161 (24.7) | |
| Inovare | 22 (2.8) | 5 (3.5) | 17 (2.6) | |
| Contrast media volume, mLb | 187 ± 110 | 175 ± 73 | 190 ± 116 | .09 |
| Q (1) (< 120 mL) | 128 (22.3) | 20 (19.6) | 108 (22.8) | .86 |
| Q (2) (120-149 mL) | 84 (14.6) | 17 (16.7) | 67 (14.2) | |
| Q (3) (150-219 mL) | 217 (37.7) | 39 (38.2) | 178 (37.6) | |
| Q (4) (≥ 220 mL) | 146 (25.4) | 26 (25.5) | 120 (25.4) | |
| Access site | .03 | |||
| Transfemoral | 743 (93.6) | 128 (89.5) | 615 (94.5) | |
| Other | 51 (6.4) | 15 (10.5) | 36 (5.5) | |
| General anesthesia | 723 (91.1) | 136 (95.1) | 587 (90.2) | .06 |
| Predilatation | 384 (48.4) | 80 (55.9) | 304 (46.7) | .04 |
| Postdilatation | 297 (37.4) | 48 (33.6) | 249 (38.2) | .29 |
| TEE guidance | 646 (81.4) | 122 (85.3) | 524 (80.5) | .18 |
| Device successc | 634 (79.8) | 90 (62.9) | 544 (83.6) | < .001 |
| Aortic regurgitationd | 54 (7.4) | 8 (6.2) | 6 (7.7) | .58 |
| Mean gradient, ≥ 20 mmHg | 30 (5.1) | 5 (4.9) | 25 (5.2) | .90 |
| Valve malpositioning | 39 (4.9) | 18 (12.6) | 21 (3.2) | < .001 |
| Multiple valve implants | 37 (4.7) | 16 (11.2) | 21 (3.2) | < .001 |
| Left ventricle perforation | 8 (1.0) | 3 (2.1) | 5 (0.8) | .16 |
| Conversion to open surgery | 7 (0.9) | 3 (2.1) | 4 (0.6) | .11 |
AKI, acute kidney injury; Other, transapical and transarterial; Q, quartiles; TEE, transesophageal echocardiogram.
Data are expressed as No. (%) or mean ± standard deviation.
Device success rate was lower and valve malpositioning was more frequently observed in patients who developed AKI following TAVI (Table 2).
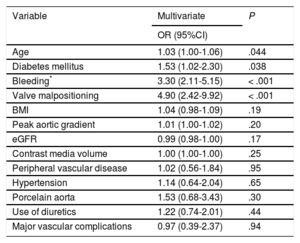
Predictors of Acute Kidney InjuryOn multivariate analysis, the independent predictors of AKI were age, diabetes mellitus, major or life-threatening bleeding, and valve malpositioning (Table 3), the latter being the strongest (odds ratio [OR], 4.90; 95% confidence interval [95%CI], 2.42-9.92; P < .001) predictor.
Multivariate Predictors of AKI in Patients Who Underwent TAVI
| Variable | Multivariate | P |
|---|---|---|
| OR (95%CI) | ||
| Age | 1.03 (1.00-1.06) | .044 |
| Diabetes mellitus | 1.53 (1.02-2.30) | .038 |
| Bleeding* | 3.30 (2.11-5.15) | < .001 |
| Valve malpositioning | 4.90 (2.42-9.92) | < .001 |
| BMI | 1.04 (0.98-1.09) | .19 |
| Peak aortic gradient | 1.01 (1.00-1.02) | .20 |
| eGFR | 0.99 (0.98-1.00) | .17 |
| Contrast media volume | 1.00 (1.00-1.00) | .25 |
| Peripheral vascular disease | 1.02 (0.56-1.84) | .95 |
| Hypertension | 1.14 (0.64-2.04) | .65 |
| Porcelain aorta | 1.53 (0.68-3.43) | .30 |
| Use of diuretics | 1.22 (0.74-2.01) | .44 |
| Major vascular complications | 0.97 (0.39-2.37) | .94 |
95%CI, 95% confidence interval; AKI, acute jidney injury; BMI, body mass index; eGFR, estimated glomerular filtration rate; OR, odds ratio; TAVI, transcatheter aortic valve implantation.
At 30 days, patients with AKI had higher rates of all-cause death (21% vs 2.9%; OR, 7.84; 95%CI, 3.70-16.58; P < .001] and cardiovascular mortality (18.2% vs 2.3%; OR, 8.55; 95%CI, 3.80-19.38; P < .001) than those without AKI. When an adjusted logistic regression model was used, AKI remained independently associated with 30-day all-cause mortality (OR, 2.71; 95%CI, 1.53-4.79; P = .001) and cardiovascular mortality (OR, 2.43; 95%CI, 1.32-4.46; P = .004).
Among patients with AKI, the 30-day all-cause death was 9.1% (OR, 3.33; 95%CI, 1.41-7.85) for those in stage 1, 36.8%(OR, 19.40; 95%CI, 6.87-54.78) in stage 2, and 41.7% (OR, 23.76; 95%CI, 10.63-53.12) in stage 3 (P < .001); 30-day cardiovascular mortality was 5.7% (OR, 2.55; 95%CI, 0.91-7.21), 36.8% (OR, 24.73; 95%CI, 8.54-71.64) and 38.9% (OR, 26.98; 95%CI, 11.61-62.71), in stages 1, 2 and 3, respectively (P < .001).
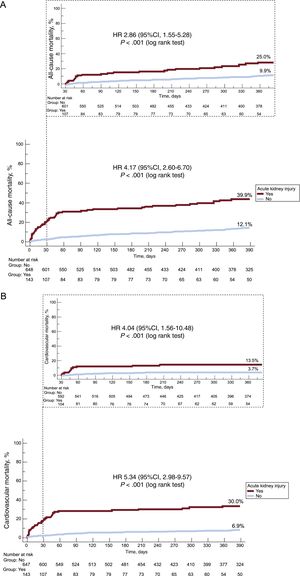
At 1-year of follow-up, we observed higher rates of all-cause (hazard ratio [HR], 4.17; 95%CI, 2.60-6.70; P < .001) and cardiovascular mortality (HR, 5.34; 95%CI, 2.98-9.57; P < .001) among patients with AKI compared with those without AKI (Figure 1).
Kaplan-Meier curves for all-cause (A) and cardiovascular mortality (B) according to acute kidney injury status during the first year of follow-up and the unadjusted landmark analysis with follow-up starting at 30 days of all-cause mortality (A) and cardiovascular mortality (B). 95%CI, 95% confidence interval; HR, hazard ratio.
When only survivors at 30 days were considered in the landmark analysis, AKI remained associated with higher risk of all-cause (HR, 2.86; 95%CI, 1.55-5.28; P < .001) and cardiovascular mortality (HR, 4.04; 95%CI, 1.56-10.48; P < .001) (Figure 1).
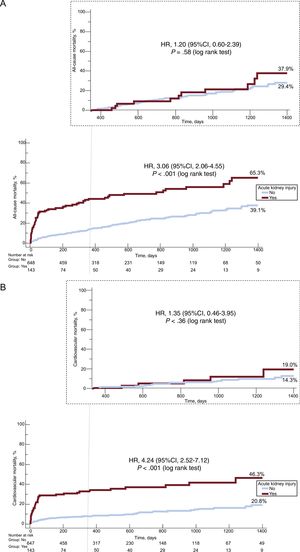
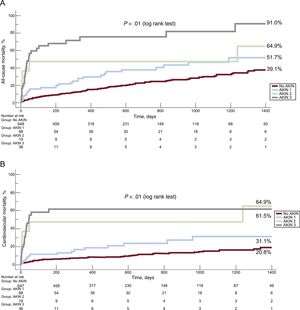
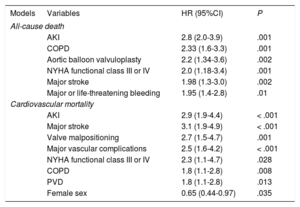
Long-term OutcomesThe presence of AKI was associated with a 3-fold increase in the risk of all-cause mortality (HR, 3.06; 95%CI, 2.06-4.55; P < .001) and a 4-fold increase in the risk of cardiovascular mortality (HR, 4.24; 95%CI, 2.52-7.12; P < .001) at 4 years of follow-up (Figure 2). Importantly, we observed a stepwise increase in all-cause and cardiovascular mortality according to different stages of AKI over the entire length of follow-up (Figure 3). Acute kidney injury remained independently associated with all-cause (adjusted HR, 2.8; 95%CI, 2.0-3.9; P < .001) and cardiovascular mortality (adjusted HR, 2.9; 95%CI, 1.9-4.4; P < .001) mortality, after adjustment for possible confounding variables (Table 4).
Kaplan-Meier curves for all-cause (A) and cardiovascular mortality (B) according to acute kidney injury status, over the entire length of follow-up and the unadjusted landmark analysis with follow-up starting at 1 year of all-cause mortality (A) and cardiovascular mortality (B). 95%CI, 95% confidence interval; HR, hazard ratio.
Predictors of Long-term All-cause Death and Cardiovascular Mortality
| Models | Variables | HR (95%CI) | P |
|---|---|---|---|
| All-cause death | |||
| AKI | 2.8 (2.0-3.9) | .001 | |
| COPD | 2.33 (1.6-3.3) | .001 | |
| Aortic balloon valvuloplasty | 2.2 (1.34-3.6) | .002 | |
| NYHA functional class III or IV | 2.0 (1.18-3.4) | .001 | |
| Major stroke | 1.98 (1.3-3.0) | .002 | |
| Major or life-threatening bleeding | 1.95 (1.4-2.8) | .01 | |
| Cardiovascular mortality | |||
| AKI | 2.9 (1.9-4.4) | < .001 | |
| Major stroke | 3.1 (1.9-4.9) | < .001 | |
| Valve malpositioning | 2.7 (1.5-4.7) | .001 | |
| Major vascular complications | 2.5 (1.6-4.2) | < .001 | |
| NYHA functional class III or IV | 2.3 (1.1-4.7) | .028 | |
| COPD | 1.8 (1.1-2.8) | .008 | |
| PVD | 1.8 (1.1-2.8) | .013 | |
| Female sex | 0.65 (0.44-0.97) | .035 | |
95%CI, 95% confidence interval; AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; HR, hazard ratio; PVD, peripheral vascular disease.
However, when considering only survivors at 12 months (428/794) in the landmark analysis, there was no difference in all-cause death (HR, 1.20; 95%CI, 0.60-2.39; P = .58) or cardiovascular mortality (HR, 1.35; 95%CI, 0.46-3.95; P = .4) among patients with and without AKI (Figure 2). Even after adjustment for important covariates, AKI was not associated with higher all-cause (adjusted HR, 1.2; 95%CI, 0.5-2.6; P = .71) and cardiovascular (adjusted HR, 0.7; 95%CI, 0.2-2.1; P = .57) mortality.
DISCUSSIONThe present study has 3 main findings: a) age, diabetes mellitus, major or life-threatening bleeding and valve malpositioning were independent predictors of AKI; b) AKI was associated with increased short- and long-term all-cause and cardiovascular mortality with a stepwise increase in mortality with higher AKI severity; and c) however, in the landmark analysis starting at 1-year of follow-up, AKI was not associated with worse outcomes, indicating that its negative impact on outcomes is limited to the first year after TAVI.
In our study, AKI was a frequent (18%) complication after TAVI, which is consistent with 2 previous meta-analyses.9,15 However, none of them included studies that used the updated VARC-2 criteria, which extends the timing for AKI diagnosis from 72hours to 7 days following TAVI. Koifman et al.,23 showed that there was approximately 10% of disagreement between the AKIN and RIFLE (Risk, Injury, Failure, Loss, End-stage) definitions, which suggests that postprocedural AKI may be misdiagnosed depending on the definition used, especially considering that peak levels of creatinine usually occur after 72hours of the procedure.13,17,23
In accordance with previous reports, our study suggests that nontransfemoral TAVI is associated with increased risk of AKI.12,17,24,25 We believe that this association is related to the higher occurrence of major vascular complications, bleeding, and need for blood transfusion with the use of alternative transarterial or transapical approaches, which are risk factors for AKI.25,26
Age, diabetes mellitus, and life-threatening bleeding are well known risk factors for the development of AKI after TAVI.10,17,27–29 However, to our knowledge, this is the first time that valve malpositioning has been shown to be an independent predictor of AKI. We suggest that the following factors may underlie this association. First, the amount of contrast media used in patients with valve malpositioning was, on average, 50% higher. Second, longer duration of the procedure, longer runs of ventricular pacing and sustained hemodynamic instability may provoke systemic inflammatory response syndrome, which appears to be a predictor of AKI and is associated with unfavorable outcomes.24,30,31 We can speculate that second-generation devices, some of which are fully repositionable and retrievable,32,33 will reduce the incidence of valve malpositioning and consequently, postprocedural AKI. Furthermore, the reduced profile of the newer delivery systems will probably reduce the rates of vascular and bleeding complications and may, therefore, also reduce the incidence of AKI.
Our findings suggest that AKI is associated with higher all-cause and cardiovascular mortality during short- (30-day landmark analysis) and long-term follow-up. However, an exploratory adjusted landmark analysis starting at 1-year of follow-up did not show differences in either all-cause death and cardiovascular mortality rates when comparing patients with and without AKI. A report by Généreux et al.,13 demonstrated that significant AKI (stages 2 or 3) was associated with an increase in short-term all-cause mortality, but not after a 30-day landmark analysis. Similarly, Barbanti et al.10 showed no significant impact of AKI on cardiovascular mortality after 30 days. Therefore, our study provides new insights as it shows that the major negative impact of AKI on mortality is limited to the first year after the procedure.
Importantly, our results showed that all stages of AKI (1, 2, and 3) had an effect on short- and long-term all-cause death and cardiovascular mortality, with a stepwise increase in mortality with higher AKI severity. This is consistent with previous findings, with even a small increase in creatinine levels (≥ 0.3mg/dL) being associated with worse prognosis.11,26 This finding highlights the clinical importance of adopting preventive measures for postprocedural AKI in a high-risk population of elderly patients undergoing TAVI.
LimitationsOur study has several limitations. First, data was self-reported by each center and, therefore, the occurrence of AKI might have been underreported, since on-site source document verification was randomly performed in only 20% of cases. Second, some patients may have been discharged from hospital earlier than 7 days; however, in our population, only 1% of the patients were discharged before this period. Third, although we appropriately adjusted for imbalance in baseline characteristics, residual confounding from unmeasured variables cannot be excluded and a cause and effect relationship between AKI and outcomes cannot be determined.
CONCLUSIONSAKI is a frequent complication following TAVI. Older age, diabetes, major or life-threatening bleeding, and valve malpositioning are independent predictors of AKI. Acute kidney injury is associated with all-cause death and cardiovascular mortality, with a stepwise increase in mortality with higher AKI severity. However, the major impact of AKI on mortality is limited to the first year after TAVI. Our results suggest that all efforts should be made to identify patients at risk, reduce procedural complications and adopt preventive measures for postprocedural AKI.
FundingSociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista, São Paulo-SP, Brazil.
CONFLICTS OF INTERESTF.S. de Brito Jr, D.A. Siqueira, and M.A. Perin are proctors from Edwards LifeSciences and Medtronic. L.A. Carvalho, and R. Sarmento-Leite are proctors from Medtronic.
- –
Acute kidney injury is frequently observed after TAVI and is associated with poorer short- and mid-term outcomes. However, there is conflicting evidence on the impact of AKI on long-term clinical outcomes.
- –
Our study provides new insights as it shows that the major negative impact of AKI on mortality is limited to the first year after the procedure.
The authors acknowledge the expert work of Patrícia O. Guimarães, Felipe N. Albuquerque, for their peer review of the manuscript, Teresa C.D.C. Nascimento, as the study coordinator and Rogério R. Prado for the statistical support.