Subclinical systolic dysfunction is one of the proposed mechanisms for increased cardiovascular risk associated with metabolic syndrome (MS). This study investigated the association between MS and impaired left ventricular global longitudinal strain (GLS) and the role of each MS criteria in this association.
MethodsWe analyzed a random sample of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) after excluding participants with prevalent heart disease.
ResultsAmong the 1055 participants fulfilling the inclusion criteria (53% women; 52±9 years), 444 (42%) had MS. Those with MS had worse GLS (–18.0%±2.5%) than those without (–19.0%±2.4%; P<.0001). In multiple linear regression models, MS was associated with worse GLS after adjustment for various risk factors (GLS difference=0.86%; P <.0001), even after inclusion of body mass index. Adjusted PR for impaired GLS as assessed by 3 cutoffs (1, 1.5, and 2 standard deviations) were higher among participants with than without MS: GLS –16.1% (PR, 1.76; 95%CI, 1.30-2.39); GLS –14.8% (PR, 2.35; 95%CI, 1.45-3.81); and GLS –13.5% (PR, 2.07; 95%CI, 0.97-4.41). After inclusion of body mass index in the models, these associations were attenuated, suggesting that they may, at least in part, be mediated by obesity. In quantile regression analyses, elevated waist circumference was the only MS component found to be independently associated with GLS across the whole range of values.
ConclusionsMetabolic syndrome is independently associated with impaired GLS. Among the MS criteria, central obesity best depicted the link between metabolic derangement and cardiac function.
Keywords
With overweight and obesity reaching epidemic proportions,1 the risks of cardiovascular morbidity and mortality have been increasingly related to adverse metabolic effects.2,3 Metabolic syndrome (MS), a cluster of cardiovascular risk factors including abdominal obesity, elevated blood pressure, impaired glucose tolerance, insulin resistance, elevated triglycerides, and low high-density lipoprotein cholesterol concentrations,4 affects about 25% of the adult population and increases the risk of heart failure,5 increased epicardial fat,6 diabetes, myocardial infarction, stroke, and death.3,7
Metabolic syndrome has been associated with systolic and diastolic dysfunction identified by tissue Doppler imaging8,9; however, ejection fraction, the most widely used parameter for evaluation of systolic function, has low sensitivity for the assessment of early dysfunction in myocardial contractility.10 New echocardiographic techniques such as global longitudinal strain (GLS) by 2-dimensional speckle-tracking echocardiography (2D-STE) allow the assessment of subclinical left ventricular dysfunction by quantitative assessment of myocardial deformation,11 although the proper GLS cutoff to define subclinical left ventricular dysfunction has not yet been established.12
Given the major obesity epidemic and related metabolic syndrome and the paucity of studies examining the impact of MS components on myocardial dysfunction,13 our aim was to investigate the association between MS and its components with impaired left ventricular GLS, using different GLS cutoffs, in middle-aged adults free of prevalent heart disease participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil).
METHODSStudy PopulationThe ELSA-Brasil is a cohort study of 15 105 men and women, civil servants from universities or research institutions, located in 6 states of Brazil (the baseline assessment was carried out from August 2008 to December 2010). All active or retired employees aged 35 to 74 years were eligible for the study. The details of the study have been described elsewhere.14,15 The ELSA-Brasil study was approved by the Research and Ethics Committees of the institutions involved, and all participants provided written informed consent. This current study investigated a predefined random sample of participants prospectively designated for ancillary studies. We included 1172 participants who underwent echocardiography with 2D-STE analysis and were evaluated for all MS components. After exclusions due to missing and/or unsuitable echocardiographic images for 2D-STE, atrial fibrillation, clinically diagnosed coronary artery disease or heart failure, or missing data on covariates, our sample was composes of 1055 participants (Figure 1).
Cardiovascular Risk Factor Assessment and Metabolic Syndrome DefinitionParticipants completed a comprehensive set of standardized medical history questionnaires determining medication use and previous clinical diagnoses. Anthropometry, blood pressure, and laboratory data were collected and analyzed following standardized protocols.14 Metabolic syndrome was defined according to the Joint Interim Statement criteria.16 Participants with at least 3 of the following criteria were considered as having MS: a) fasting plasma glucose level ≥ 100mg/dL (5.5 mmol/L) or medication use for hyperglycemia; b) raised triglyceride concentrations ≥ 150mg/dL (1.7 mmol/L) or specific treatment for this lipid anomaly; c) reduced high-density lipoprotein cholesterol concentrations <40mg/dL (1.0 mmol/L) for men and< 50mg/dL (1.3 mmol/L) for women, or specific treatment for this lipid anomaly; d) abdominal obesity, as per the European origin definition (waist circumference ≥ 94cm for men and ≥ 80cm for women); and e) blood pressure ≥ 130/85mmHg, or treatment for hypertension.
Two-dimensional EchocardiographyAll echocardiograms were performed by trained cardiologists following recommendations by the European and North American Cardiology Societies.17,18 All studies were obtained using identical equipment (Aplio XG, Toshiba Corporation, Toshigi, Japan) with a 2.5MHz sector transducer. Sequences of 3 consecutive heartbeats in each echocardiographic window were selected and recorded in digital format and were then transferred to the ELSA-Brasil echocardiography reading center, together with an image acquisition form reporting image quality and preliminary findings assessment. All studies were blindly read for standard echocardiographic parameters at the reading center, under a prespecified protocol in a dedicated workstation (ComPACS Review Station 10.5, Medimatic Solutions Srl, Italy).14
Left Ventricular Global Longitudinal StrainThe quantitative assessment of myocardial deformation followed current procedures and guidelines for STE,11,18 using commercially available software (2D Cardiac Performance Analysis, TomTec-Arena 1.2 Imaging Systems, Unterschleißheim, Germany). For analysis, endocardial borders were traced at the end-diastolic frame in the apical 2- and 4-chamber views. End-diastole was delimited by the QRS complex or the frame after mitral valve closure. The 2D-STE software tracks speckle patterns along the endocardial border throughout the cardiac cycle. Subsequently, GLS was measured and computed automatically (in 6 segments from each view) and are presented as peak average proportional shortening in percentage. The reproducibility of GLS measurements was evaluated in a sample of 50 randomly selected participants. Intra- and interobserver coefficients of variation were 5.4% and 7.4%, and intraclass correlation coefficients were 0.86 (95%CI, 0.77-0.92) and 0.76 (95%CI: 0.61-0.86).
Statistical AnalysisData are presented as mean±standard deviation for continuous variables and as total number and proportion for categorical variables. The Student t test was used to assess differences in continuous variables between the groups studied, whereas the Fisher and chi-square tests were used for categorical analysis. Multiple linear regression analysis was performed to adjust for potential confounders in the relationship between MS and GLS. The association between MS and categorically-defined impaired GLS was tested with robust Poisson regression19 using 3 proposed cutoffs for GLS.12 Taking into consideration the uncertainty about the most appropriate GLS threshold, we also investigated nonlinear associations of MS and its individual components across different GLS thresholds (dependent variable) using quantile regression analysis.20 Differences in strain (in % GLS units) related to the presence of MS components were estimated across the range of GLS distribution (5th to 95th quantiles). Multivariable models were adjusted for clinically relevant covariates identified in the literature, removing from all regression models those variables that did not alter the association between MS and GLS. All tests were 2-sided, and P values< .05 were considered statistically significant. Statistical analyses were performed with SAS 9.4 (SAS Institute, Inc, Cary, North Carolina, United States).
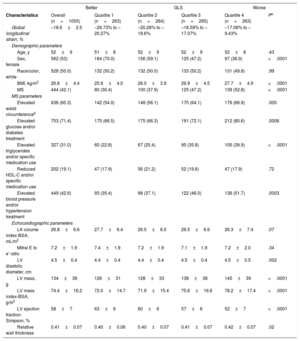
RESULTSFrom our analytic sample of 1055 participants (53% women; 52±9 years), 444 (42%) fulfilled the diagnostic criteria for MS. The clinical and demographic characteristics of all participants according to GLS quartiles are shown in Table 1. Worse GLS (less negative values) was associated with an increased prevalence of MS and its components, except for low high-density lipoprotein cholesterol (all other P values <.001). The baseline sociodemographic characteristics between the data of this random sample and the available data published in the cohort profile of ELSA-Brasil were similar and are shown in Table 1 of the supplementary material.
Clinical and Demographic Characteristics of Participants According to GLS Quartiles. Cohort Random Sample, the ELSA-Brasil Study (2008-2012) (N=1055)
| Better | GLS | Worse | ||||
|---|---|---|---|---|---|---|
| Characteristics | Overall (n=1055) | Quartile 1 (n=263) | Quartile 2 (n=264) | Quartile 3 (n=265) | Quartile 4 (n=263) | Pa |
| Global longitudinal strain, % | –18.6±2.5 | –26.73% to –20.27% | –20.26% to –18.6% | –18.59% to –17.07% | –17.06% to –9.43% | |
| Demographic parameters | ||||||
| Age, y | 52±9 | 51±8 | 52±9 | 52±9 | 52±8 | .43 |
| Sex, female | 562 (53) | 184 (70.0) | 156 (59.1) | 125 (47.2) | 97 (36.9) | <.0001 |
| Race/color, white | 528 (50.0) | 132 (50.2) | 132 (50.0) | 133 (50.2) | 131 (49.8) | .99 |
| BMI, kg/m2 | 26.6±4.4 | 25.9±4.0 | 26.0±3.9 | 26.9±4.5 | 27.7±4.9 | <.0001 |
| MS | 444 (42.1) | 80 (30.4) | 100 (37.9) | 125 (47.2) | 139 (52.8) | <.0001 |
| MS parameters | ||||||
| Elevated waist circumferenceb | 636 (60.3) | 142 (54.0) | 148 (56.1) | 170 (64.1) | 176 (66.9) | .005 |
| Elevated glucose and/or diabetes treatment | 753 (71.4) | 175 (66.5) | 175 (66.3) | 191 (72.1) | 212 (80.6) | .0006 |
| Elevated triglycerides and/or specific medication use | 327 (31.0) | 60 (22.8) | 67 (25.4) | 95 (35.8) | 105 (39.9) | <.0001 |
| Reduced HDL-C and/or specific medication use | 202 (19.1) | 47 (17.9) | 56 (21.2) | 52 (19.6) | 47 (17.9) | .72 |
| Elevated blood pressure and/or hypertension treatment | 449 (42.6) | 93 (35.4) | 98 (37.1) | 122 (46.0) | 136 (51.7) | .0003 |
| Echocardiographic parameters | ||||||
| LA volume index-BSA, mL/m2 | 26.8±6.6 | 27.7±6.4 | 26.5±6.0 | 26.5±6.6 | 26.3±7.4 | .07 |
| Mitral E to e’ ratio | 7.2±1.9 | 7.4±1.9 | 7.2±1.9 | 7.1±1.9 | 7.2±2.0 | .34 |
| LV diastolic diameter, cm | 4.5±0.4 | 4.4±0.4 | 4.4±0.4 | 4.5±0.4 | 4.5±0.5 | .002 |
| LV mass, g | 134±36 | 126±31 | 128±33 | 138±38 | 145±39 | <.0001 |
| LV mass index-BSA, g/m2 | 74.4±16.2 | 72.0±14.7 | 71.9±15.4 | 75.6±16.6 | 78.2±17.4 | <.0001 |
| LV ejection fraction-Simpson, % | 58±7 | 63±6 | 60±6 | 57±6 | 52±7 | <.0001 |
| Relative wall thickness | 0.41±0.07 | 0.40±0.06 | 0.40±0.07 | 0.41±0.07 | 0.42±0.07 | .02 |
BMI, body mass index; BSA, body surface area; GLS, global longitudinal strain; HDL-C, high-density lipoprotein cholesterol; LA, left atrium; LV: left ventricle; MS, metabolic syndrome.
The values are expressed as mean±standard deviation or No. (%).
In the overall sample, the mean GLS was –18.6%±2.5%. Individuals with MS had worse left ventricular systolic function as measured by GLS (–18.0%±2.5%) compared with participants without MS (–19.0%±2.4%, P <.0001). On multiple linear regression analysis (Table 2), the presence of MS was associated with a 0.86 absolute reduction in GLS (GLS in percentage units, P <.0001) after adjustment for age, sex, race/color, educational level, and study center. This absolute GLS impairment with MS was attenuated to 0.60 (GLS percentage units) after adjustment for body mass index, but remained highly statistically significant (P=.0005).
Unadjusted and Adjusted Absolute Worsening in GLS (percentage units) With the Presence of Metabolic Syndrome. Cohort Random Sample, the ELSA-Brasil Study (2008-2012) (N=1055)
| Worsening in GLS | ||
|---|---|---|
| GLS percentage units (SE) | P | |
| Model 1 | 0.99 (0.15) | <.0001 |
| Model 2 | 0.86 (0.15) | <.0001 |
| Model 3 | 0.60 (0.17) | .0005 |
GLS, global longitudinal strain; SE: standard error.
Adjusted through multiple linear regression for the following:
Model 1: crude. [R-Square (R2): 4%].
Model 2: model 1+sex, age (years), race/color, educational level, ELSA-Brasil center. (R2: 13%).
Model 3: model 2+body mass index (kg/m2). (R2: 14%).
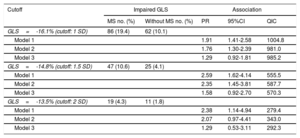
Similarly, we found about twice the prevalence of subclinical systolic dysfunction among participants with MS (Table 3, model 2), corresponding to higher adjusted prevalence ratios (PR) for impaired GLS as estimated with robust Poison regression. Using increasingly more stringent cutoffs for GLS (1, 1.5 and 2 standard deviations [SD]), the associations were as follows: for GLS –16.1% (PR, 1.76; 95%CI, 1.30-2.39), for GLS –14.8% (PR, 2.35; 95%CI, 1.45-3.81), and for GLS –13.5% cut-point (PR, 2.07; 95%CI, 0.97-4.41). After inclusion of body mass index in the model, associations were attenuated and were no longer statistically significant (Table 3).
Unadjusted and Adjusted Prevalence Ratios for the Association of Metabolic Syndrome With Impaired GLS Defined by Various Cutoffs. Cohort Random Sample, the ELSA-Brasil Study (2008-2012) (N=1055)
| Cutoff | Impaired GLS | Association | |||
|---|---|---|---|---|---|
| MS no. (%) | Without MS no. (%) | PR | 95%CI | QIC | |
| GLS=-16.1% (cutoff: 1 SD) | 86 (19.4) | 62 (10.1) | |||
| Model 1 | 1.91 | 1.41-2.58 | 1004.8 | ||
| Model 2 | 1.76 | 1.30-2.39 | 981.0 | ||
| Model 3 | 1.29 | 0.92-1.81 | 985.2 | ||
| GLS=-14.8% (cutoff: 1.5 SD) | 47 (10.6) | 25 (4.1) | |||
| Model 1 | 2.59 | 1.62-4.14 | 555.5 | ||
| Model 2 | 2.35 | 1.45-3.81 | 587.7 | ||
| Model 3 | 1.58 | 0.92-2.70 | 570.3 | ||
| GLS=-13.5% (cutoff: 2 SD) | 19 (4.3) | 11 (1.8) | |||
| Model 1 | 2.38 | 1.14-4.94 | 279.4 | ||
| Model 2 | 2.07 | 0.97-4.41 | 343.0 | ||
| Model 3 | 1.29 | 0.53-3.11 | 292.3 | ||
95%CI, 95% confidence interval; GLS, global longitudinal strain; MS, metabolic syndrome; SD, standard deviation; PR, prevalence ratio; QIC, quasilikelihood under the independence model criterion.
Model 1: metabolic syndrome.
Model 2: model 1+sex, age (years), race/color, educational level, ELSA-Brasil center.
Model 3: model 2+body mass index (kg/m2).
Figure 2 shows quantile regression differences in strain (in GLS percentage units) between participants with and without MS or with its components across the range of GLS distribution (5th to 95th quantiles), when adjusted for age, sex, race/color, educational level and study center, and in the individual components analyses additionally for the remaining MS criteria. We observed a significant difference in strain in participants with MS, which appeared to increase with worsening GLS (Figure 2). Among individual MS components, only elevated waist circumference was associated with poorer GLS. As in the other analyses, these associations were mostly attenuated when body mass index was included in the models (Table 2 of the supplementary material).
Adjusted quantile regression differences in GLS (in GLS percentage units and 95% confidence intervals) related to the presence of metabolic syndrome and of its components across the 5th and 95th quantiles of GLS. Adjusted for sex, age (years), race/color, educational level and ELSA-Brasil center, and in analyses of individual components of metabolic syndrome additionally for the remaining metabolic syndrome components. GLS, global longitudinal strain; HDL, high-density lipoprotein.
The variables included in the initial investigation and removed from all final regression models, as they did not modify the association of MS with GLS, were heart rate, left ventricular mass, mitral E to e’ ratio, left ventricular diastolic diameter, and ejection fraction. Despite this, we also investigated the role of ejection fraction as a potential confounder in the association of MS with GLS. In linear regression, when we added 2D-STE left ventricular ejection fraction in the model, the association remained statistically significant (GLS difference 0.62%; P <.0001). The ejection fraction only minimally affected the size and significance of the association between MS and GLS in robust Poisson regression when the same GLS cutoffs were used in 3 separate models (1 SD: PR, 1.49; 95%CI, 1.12-1.97; 1.5 SD: PR, 1.95; 95%CI, 1.25-3.02; and 2 SD: PR, 1.66; 95%CI, 0.85-3.23).
DISCUSSIONIn this multicenter cohort of middle-aged individuals, one of the largest reported MS samples of nonreferred participants, we investigated the association of MS and its components with GLS, an indicator of subclinical systolic dysfunction. Participants with MS (42%) had worse left ventricular systolic function assessed by different GLS parameters than those without MS. Participants with MS had about twice the prevalence of subclinical systolic dysfunction, using different GLS cutoff points. MS was independently associated with impaired GLS. After inclusion of body mass index in the models, the associations diminished somewhat, suggesting that, at least in part, they are explained by obesity. Furthermore, on analysis of each individual component of MS, elevated waist circumference was the only component that remained independently associated with impaired GLS.
In 2009, major health organizations proposed consensus criteria for the clinical diagnosis of MS.16 However, as we have shown, different components have distinct associations with GLS.13 Thus, it may make more sense to identify the association of impaired GLS with MS components than with the resultant, more complex definition.21–25
The associations of MS with myocardial dysfunction have been previously shown in smaller samples using conventional echocardiography, tissue Doppler imaging,8,9 and STE.13 However, few studies using 2D-STE have examined which components of the MS are independently associated with the impairment of systolic function. Previous studies demonstrated the unfavorable influence of different MS risk factors on left ventricular deformation such as hypertension,22 diabetes,23 obesity,24 and dyslipidemia.25
In addition, some investigations have demonstrated the existence of stronger associations between the components of MS and myocardial dysfunction with an increasing number of MS risk factors. Tadic et al.26 showed that an increasing number of MS criteria is associated with cardiac diastolic dysfunction. In the Multiethnic Study of Atherosclerosis,27 waist circumference and fasting glucose were significantly associated with left ventricular impairment of circumferential and longitudinal strain, respectively. That study also showed that GLS changed from –14.2% in participants with ≤ 1 MS component, to –13.4%, in participants with 2, and to –12.1% in those with ≥ 3 MS criteria (P <.01). Recently, Tadic et al.28 and Wang et al.29 found that among all MS components, blood pressure, waist circumference, and fasting plasma glucose were the components most closely associated with left ventricular deformation indices. Obesity and cardiometabolic risk factors have been implicated in myocardial dysfunction such as coronary dysfunction and diabetic cardiomyopathy.30,31 Nonetheless, the mechanisms contributing to these changes remain incompletely understood, and are believed to be the result of a complex interplay of hemodynamic and neurohumoral factors as well as of inflammation and oxidative stress, which contribute to cellular apoptosis, hypertrophy, and interstitial fibrosis.30-32
The 2D-STE has emerged as a more robust technique to detect subclinical left ventricular dysfunction by quantitative assessment of myocardial deformation.11 This technique has the advantages of angle independency, and less dependency on loading conditions and geometry than traditional parameters. Additionally, it permits postprocessing in regular 2D acquired images, thus making highly feasible the reproducible examination of a major component of regional myocardial performance. The 2D-STE is becoming widely available, due to technological development and its inclusion in most commercially available echocardiographic systems. Despite speckle-tracking software standardization concerns,11,33 the distribution of GLS values in our study was similar to that found in a recent meta-analysis,12 as was its measurement variability,34 which supports the quality of our data.
Limitations and StrengthsTo overcome the limitation of current uncertainty about which GLS cutoff definition is most relevant to define subclinical left ventricular systolic impairment, we explored the associations of MS with 3 different reference cutoffs for GLS. Our findings demonstrate a high prevalence of impaired GLS in MS participants (19.3%) compared with the rest of the sample (10.1%) using the less conservative 1 SD cutoff. This higher prevalence was independently present after adjustment for relevant covariates, with the exception of body mass index. As expected, prevalences were lower when stricter cutoffs were used, but associations were in general similar. The 1.5 SD cutoff is closer to a “15% relative percentage reduction in GLS”, which has recently been considered a relevant cutoff to define cardiotoxicity by many experts.35 The lack of statistical significance of impaired GLS categorically defined by the 2 SD cutoff may be a consequence of the fact that very few participants had values altered to this extent. Of note, the associations between MS and its components were present with different left ventricular GLS thresholds, which would imply that MS has a progressive impact on a wide range of left ventricular functions from subclinical stages. Of interest, in the present study, all the parameters of MS, except reduced high-density lipoprotein cholesterol, were individually associated with impaired GLS (all P-values <.01). In addition, after adjusting for most of confounders, we demonstrated that elevated waist circumference was the only MS definition criteria independently associated with impaired GLS across the whole range of GLS distribution (5th to 95th quantiles). Our findings are in consonance with the results of a recent study36 showing that the presence of abdominal adiposity was associated with lower GLS, both in participants with and without general obesity. The concept of “metabolically healthy obesity”, characterized by preserved insulin sensitivity, relatively low visceral fat mass, favorable cholesterol levels, and normal blood pressure, is currently in vogue.37 Although persons who are “metabolically unhealthily obese” appear to be more prone to complications, recent studies have shown that obesity was associated with differences in both subclinical systolic and diastolic function, regardless of “health” as defined by the presence or absence of MS.38,39
The strengths of this study are the large number of participants randomly selected from a multicenter cohort and the use of advanced imaging techniques. However, our study also has limitations. Because of the cross-sectional design of our study, a causal relationship between MS and its components with GLS cannot be determined. Prospective studies with a large number of participants are essential to evaluate the impact of impaired left ventricular deformation on cardiovascular morbidity and mortality in persons with MS. Although we accounted for several confounders and performed multivariate analyses adjusting for established cardiovascular risk factors, we cannot rule out the possibility of unmeasured confounders playing a role in the observed associations. Finally, the most appropriate GLS cutoff to define abnormality is still unclear. For this reason, we transformed this theoretical limitation into a methodological strength, using an approach to test GLS continuously.
CONCLUSIONSLeft ventricular function assessed by GLS was significantly impaired in MS participants, and the prevalence of impaired left ventricular GLS was higher in MS participants at several different GLS cutoffs. Moreover, we identified elevated waist circumference as the main MS component associated with impaired left ventricular GLS. Among the MS criteria, central obesity best depicted the link between metabolic derangement and cardiac function.
FUNDINGThis work was supported by the Brazilian Ministry of Health (Science and Technology Department), the Brazilian Ministry of Science, Technology and Innovation (Financiadora de Estudos e Projetos-grants 01 06 0010.00, 01 10 0643.00 RS, 01 06 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00 SP, 01 06 0071.00 RJ), CNPq (the Brazilian National Council for Scientific and Technological Development), and Coordination for the Improvement of Higher Education Personnel-CAPES (grants 1407742 [W. Cañon-Montañez] and 1556322 [M. Bessel]).
- –
Metabolic syndrome is a clustering of cardiometabolic risk factors, including abdominal obesity, impaired glucose tolerance, insulin resistance, dyslipidemia, and hypertension.
- –
Metabolic syndrome affects about 25% of the adult population, and increases the risk of heart failure, diabetes, myocardial infarction, stroke, and death.
- –
Subclinical systolic dysfunction is one of the proposed mechanisms for increased cardiovascular risk associated with MS and can be detected by left ventricular GLS using 2D-STE.
- –
This study explored the associations of MS with 3 different reference cutoffs for GLS.
- –
Metabolic syndrome was independently associated with impaired left ventricular GLS.
- –
Among the MS criteria, central obesity best depicted the link between metabolic derangement and cardiac function.
None declared.
The authors would like to acknowledge all ELSA-Brasil participants for their valuable contribution to this study.