Little is known about the timing of onset and outcome of conduction abnormalities (CA) following balloon-expandable transcatheter aortic valve implantation. The aim of this study was to examine the timing of CA and determine the impact of balloon aortic valvuloplasty (BAV) on the persistence of these abnormalities.
MethodsA total of 347 patients were included. Of these, 75 had a continuous electrocardiogram recording and a 6-lead electrocardiogram at each step of the procedure.
ResultsIn the transcatheter aortic valve implantation population undergoing continuous electrocardiogram monitoring, new-onset left bundle branch block (LBBB) or third-degree atrioventricular block occurred in 48 (64%) and 16 (21.3%) patients, with 51.5% of CA occurring before valve implantation. Left bundle branch block persisted more frequently at hospital discharge (53.8 vs 22.7%; P=.028) and at 1-month follow-up (38.5 vs 13.6%; P=.054) when occurring before valve implantation. Balloon aortic valvuloplasty prior to valve implantation was used in 264 (76.1%) patients, and 78 (22.5%) had persistent LBBB or complete atrioventricular block requiring pacemaker implantation. Persistent LBBB or unresolved atrioventricular block at 1 month occurred more frequently in the BAV group (76.1 vs 47.6%; P=.021), and the use of BAV was associated with a lack of CA resolution (OR, 3.5; 95%CI, 1.17-10.43; P=.021).
ConclusionsIn patients undergoing a balloon-expandable transcatheter aortic valve implantation, more than half of CA occurred before valve implantation. Early occurrence of CA was associated with a higher rate of persistence at 1-month follow-up. The use of BAV was associated with an increased risk of CA persistence.
Keywords
The continual development of transcatheter aortic valve implantation (TAVI) has significantly reduced procedural complications.1 Nevertheless, conduction abnormalities (CA) remain frequent and their incidence is unlikely to decrease in the near future.1 In fact, an increased rate of CA has been reported with some of the newer generation transcatheter valves.1 Predictive factors of CA for both balloon- and self-expanding transcatheter prostheses have been elucidated.2,3 However, little is known of the timing and mechanisms underlying the occurrence of CA during TAVI. While up to 50% of CA occurred during balloon valvuloplasty or wire manipulation during TAVI with self-expanding prostheses, these issues have not been systematically evaluated within the context of TAVI with balloon-expandable prostheses.4 Moreover, a significant number of CA following balloon-expandable valve implantation are transient and resolve during the hospitalization period, or within the first few weeks following the procedure.5–7 Whether the mechanisms and timing of CA ultimately impact the resolution and persistence of CA over time remains unknown. Recent studies have shown that performing TAVI without prior balloon predilation (balloon aortic valvuloplasty [BAV]) is feasible and safe and may have benefits in terms of procedural duration and contrast dose.8,9 Although the impact of balloon predilation has not been completely elucidated, some authors have suggested that moderate BAV may reduce the risk of permanent pacemaker (PPM) implantation.10 However, it is unknown whether direct TAVI (ie, without balloon predilation) decreases the risk and, more importantly, whether CA persist over time. Therefore, the objectives of this study were: a) to evaluate the timing of onset and outcome of CA in patients undergoing TAVI with a balloon-expandable valve, and b) to determine the impact of BAV on the occurrence and persistence of CA post-TAVI.
METHODSStudy PopulationA total of 707 patients underwent TAVI at the 2 participating centers between May 2010 and December 2015, and 554 of them underwent a balloon-expandable valve implantation. Exclusion criteria consisted of self-expandable valve implantation (n=153), a pre-existing PPM (n=82), left bundle branch block (LBBB) (n=41), right bundle branch block (n=52), transient CA detected during a 24-hour electrocardiogram monitoring prior to TAVI (n=9), and a valve-in-valve procedure (n=23). The final study population consisted of 347 patients. Continuous electrocardiogram recording during the procedure was performed in a subgroup of 75 consecutive patients between February 2013 and November 2015 who underwent a TAVI procedure including BAV step in 1 of the 2 study centers.
Indications for TAVI and the approach were determined by local Heart Teams and data were prospectively gathered in local datasets. Outcomes were defined according to the Valve Academic Research Consortium-2 criteria.11 Patients signed informed consent forms before the procedure.
Transcatheter Aortic Valve Implantation Procedure and Balloon Aortic ValvuloplastyThe TAVI procedures were performed with the balloon-expandable valve SAPIEN XT or SAPIEN 3 (Edwards Lifesciences, Irvine, California, United States) as previously reported.12,13 The decision to perform BAV prior to valve implantation was left at operator's discretion. In the case of BAV, moderate BAV under rapid pacing with an undersized balloon was used in all cases.
Continuous Electrocardiogram Monitoring and Electrocardiogram Data AnalysisA 12-lead electrocardiogram was obtained upon hospital admission in all patients. Continuous electrocardiogram recording during TAVI procedures was carried-out using a 2-limb lead electrocardiogram. In addition, in a subgroup of 75 consecutive patients undergoing TAVI in 1 of the 2 study centers, a 6-limb lead electrocardiogram was recorded immediately before TAVI, and immediately after each of the following procedural steps: placement of the stiff-wire in the left ventricle, BAV, valve implantation, and valve postdilation or implantation of a second valve if needed. In addition, any new CA was recorded at the time of occurrence (any time during the procedure). A 12-lead electrocardiogram was recorded immediately post-TAVI and daily until hospital discharge and all patients were under continuous electrocardiogram monitoring for at least 72hours postprocedure.
All electrocardiograms were prospectively analyzed by a cardiologist at each center. Diagnosis of new conduction disturbances was based on the recommendations of the American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society for the standardization and interpretation of electrocardiograms.14 Indications for PPM were in accordance with current recommendations.15 Transient new-onset LBBB was defined as a new LBBB that resolved before the 1-month follow-up. Atrioventricular block (AVB) resolution in patients receiving a PPM was defined as a ventricular pacing<1% at 1-month follow-up.16
Follow-upPatients were followed up through outpatient visits at 1-month follow-up. A 12-lead electrocardiogram was obtained in all patients. In addition, ventricular pacing rates were evaluated in patients receiving a PPM post-TAVI.
Statistical AnalysisData are expressed as mean±standard deviation or median [interquartile range] for continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared using the 2-sided Student t test and Mann-Whitney U test, according to the normality of the distribution. Categorical variables were compared using the chi-square or Fisher exact test as appropriate. A P value<.05 was considered significant. Analyses were conducted using the Statistical Package for Social Sciences version 22 (SPSS Inc, IBM, Armonk, New York, United States).
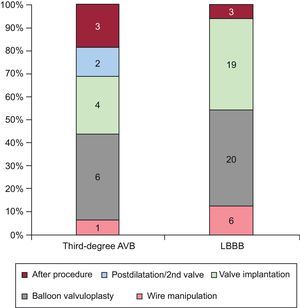
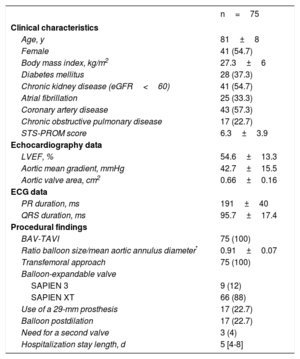
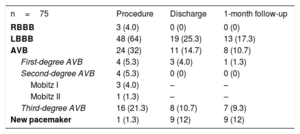
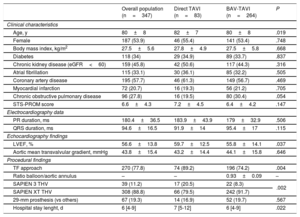
RESULTSTiming of Conduction AbnormalitiesThe main baseline characteristics of the 75 patients with continuous electrocardiogram procedural monitoring/recording are displayed in Table 1. A total of 75 CA occurred in 58 (77.3%) patients during the hospitalization period. The types of CA are detailed in Table 2. New-onset LBBB occurred in 48 (64%) patients and a third-degree AVB in 16 (21.3%) patients. The timing of onset of LBBB and third-degree AVB are shown in Figure 1. Up to 54.2% of new-onset LBBB and 43.7% of third-degree AVB episodes occurred before valve implantation.
Main Clinical, Echocardiographic and Procedural Characteristics of Patients Undergoing ECG Monitoring and Systematic ECG Recording During TAVI
| n=75 | |
|---|---|
| Clinical characteristics | |
| Age, y | 81±8 |
| Female | 41 (54.7) |
| Body mass index, kg/m2 | 27.3±6 |
| Diabetes mellitus | 28 (37.3) |
| Chronic kidney disease (eGFR<60) | 41 (54.7) |
| Atrial fibrillation | 25 (33.3) |
| Coronary artery disease | 43 (57.3) |
| Chronic obstructive pulmonary disease | 17 (22.7) |
| STS-PROM score | 6.3±3.9 |
| Echocardiography data | |
| LVEF, % | 54.6±13.3 |
| Aortic mean gradient, mmHg | 42.7±15.5 |
| Aortic valve area, cm2 | 0.66±0.16 |
| ECG data | |
| PR duration, ms | 191±40 |
| QRS duration, ms | 95.7±17.4 |
| Procedural findings | |
| BAV-TAVI | 75 (100) |
| Ratio balloon size/mean aortic annulus diameter* | 0.91±0.07 |
| Transfemoral approach | 75 (100) |
| Balloon-expandable valve | |
| SAPIEN 3 | 9 (12) |
| SAPIEN XT | 66 (88) |
| Use of a 29-mm prosthesis | 17 (22.7) |
| Balloon postdilation | 17 (22.7) |
| Need for a second valve | 3 (4) |
| Hospitalization stay length, d | 5 [4-8] |
BAV, balloon aortic valvuloplasty; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; TAVI, transcatheter aortic valve implantation.
Values are expressed as no. (%), mean±standard deviation or median [interquartile range].
New Conduction Abnormalities Related to the TAVI Procedure, and Persistent Conduction Disturbances at Discharge and 1-month Follow-up
| n=75 | Procedure | Discharge | 1-month follow-up |
|---|---|---|---|
| RBBB | 3 (4.0) | 0 (0) | 0 (0) |
| LBBB | 48 (64) | 19 (25.3) | 13 (17.3) |
| AVB | 24 (32) | 11 (14.7) | 8 (10.7) |
| First-degree AVB | 4 (5.3) | 3 (4.0) | 1 (1.3) |
| Second-degree AVB | 4 (5.3) | 0 (0) | 0 (0) |
| Mobitz I | 3 (4.0) | – | – |
| Mobitz II | 1 (1.3) | – | – |
| Third-degree AVB | 16 (21.3) | 8 (10.7) | 7 (9.3) |
| New pacemaker | 1 (1.3) | 9 (12) | 9 (12) |
AVB, atrioventricular block, LBBB, left bundle branch block, RBBB, right bundle branch block; TAVI, transcatheter aortic valve implantation.
Values are expressed as no. (%).
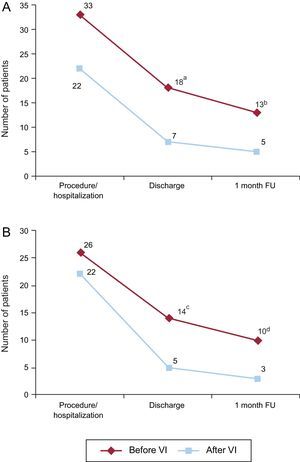
Persistent new-onset LBBB or unresolved AVB requiring PPM implantation before hospital discharge occurred in 25 of 75 patients, 46.3% of patients with a new LBBB or third-degree AVB related to the TAVI procedure. The occurrence of CA before valve implantation (during wire manipulation or BAV) tended to be associated with a higher rate of persistent LBBB or unresolved AVB at hospital discharge (54.5 vs 31.8%, P=.097). At 1-month follow-up, 18 out of 74 patients (24.3%) with complete follow-up (33.3% of patients with new-onset LBBB or third-degree AVB) had a persistent LBBB or unresolved AVB with a ventricular pacing>1% of the time. The occurrence of CA before valve implantation was not associated with a higher rate of persistent CA at 1-month follow-up (39.4 vs 23.8%; P=.236) (Figure 2A).
Persistence of conduction abnormalities over time. Number of patients with new-onset LBBB or unresolved AVB (A) and new-onset LBBB (B) at each time point according to its onset before or after valve implantation. AVB, atrioventricular block, FU, follow-up; LBBB, left bundle branch block, VI, valve implantation. aP=.097. bP=.236. cP=.028. dP=.054.
Up to 60.4% of new LBBB occurring during the procedural time resolved during the hospitalization period, most of them (86.2%) within the first 24hours following TAVI. Among the 19 (39.6%) patients with persistent LBBB upon hospital discharge, in 6 (31.6%) patients there was LBBB resolution at 1-month follow-up. The persistence of LBBB over time according to the timing of onset (before or after valve implantation) is shown in Figure 2B. LBBB persisted more frequently at hospital discharge (53.8 vs 22.7%; P=.028) and at the 1 month follow-up (38.5 vs 13.6%; P=.054) when occurring before valve implantation.
The onset of the new CA occurred after the procedure in 6 patients: 3 (18.75%) third-degree AVB and 3 (6.25%) new LBBB. These third-degree AVBs occurred during the first 24hours after the procedure, and 2 of them were persistent at 30-days follow-up. In the 3 patients with a new LBBB after the procedure, only 1 of them occurred after the first 24hours. In 2 patients, the LBBB was associated with other CA leading to PPM implantation and showed persistent LBBB or unresolved AVB at 30-days follow-up.
Nine patients received a PPM during the hospitalization period. The reasons for PPM were complete AVB in 8 (88.8%) patients and new-onset LBBB and first-degree AVB in 1 patient. No differences were observed in the rate of unresolved AVB requiring PPM implantation regarding its timing of onset (57.1% before vs 44.4% after valve implantation; P=.50). All second-degree AVBs were transient. At 1-month follow-up, the mean rate of ventricular pacing in patients requiring PPM before hospital discharge was of 82.7±34.5%. Atrioventricular block resolution with a ventricular pacing<1% occurred in 1 patient, without differences between those occurring before and those after valve implantation (25% vs 0%; P=.50).
Impact of Balloon Aortic Valvuloplasty on the Persistence of Conduction AbnormalitiesA total of 264 out of 347 (76.1%) patients underwent TAVI with BAV preceding valve implantation, and 83 (23.9%) patients underwent direct TAVI. The main baseline clinical characteristics and echocardiographic and procedural findings of the study population according to the use of BAV are shown in Table 3. Patients in the direct TAVI group were older (82±7 vs 80±8 years; P=.019), had a higher left ventricular ejection fraction (60±13 vs 56±14%; P=.037), underwent TAVI more frequently using the transfemoral approach (89.2 vs 74.3%; P=.004), and more frequently received a SAPIEN 3 valve (20.5 vs 8.3%; P=.002).
Baseline and Procedural Characteristics of the Study Population According to the Use of BAV During the TAVI Procedure
| Overall population (n=347) | Direct TAVI (n=83) | BAV-TAVI (n=264) | P | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 80±8 | 82±7 | 80±8 | .019 |
| Female | 187 (53.9) | 46 (55.4) | 141 (53.4) | .748 |
| Body mass index, kg/m2 | 27.5±5.6 | 27.8±4.9 | 27.5±5.8 | .668 |
| Diabetes | 118 (34) | 29 (34.9) | 89 (33.7) | .837 |
| Chronic kidney disease (eGFR<60) | 159 (45.8) | 42 (50.6) | 117 (44.3) | .316 |
| Atrial fibrillation | 115 (33.1) | 30 (36.1) | 85 (32.2) | .505 |
| Coronary artery disease | 195 (57.7) | 46 (61.3) | 149 (56.7) | .469 |
| Myocardial infarction | 72 (20.7) | 16 (19.3) | 56 (21.2) | .705 |
| Chronic obstructive pulmonary disease | 96 (27.8) | 16 (19.5) | 80 (30.4) | .054 |
| STS-PROM score | 6.6±4.3 | 7.2±4.5 | 6.4±4.2 | .147 |
| Electrocardiography data | ||||
| PR duration, ms | 180.4±36.5 | 183.9±43.9 | 179±32.9 | .506 |
| QRS duration, ms | 94.6±16.5 | 91.9±14 | 95.4±17 | .115 |
| Echocardiography findings | ||||
| LVEF, % | 56.6±13.8 | 59.7±12.5 | 55.8±14.1 | .037 |
| Aortic mean transvalvular gradient, mmHg | 43.8±15.4 | 43.2±14.4 | 44.1±15.8 | .646 |
| Procedural findings | ||||
| TF approach | 270 (77.8) | 74 (89.2) | 196 (74.2) | .004 |
| Ratio balloon/aortic annulus | – | – | 0.93±0.09 | – |
| SAPIEN 3 THV | 39 (11.2) | 17 (20.5) | 22 (8.3) | .002 |
| SAPIEN XT THV | 308 (88.8) | 66 (79.5) | 242 (91.7) | |
| 29-mm prosthesis (vs others) | 67 (19.3) | 14 (16.9) | 52 (19.7) | .567 |
| Hospital stay lenght, d | 6 [4-9] | 7 [5-12] | 6 [4-9] | .022 |
BAV, balloon aortic valvuloplasty; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; TAVI, transcatheter aortic valve implantation; TF, transfemoral; THV, transcatheter heart valve.
Values are expressed as no. (%), mean±standard deviation or median [interquartile range].
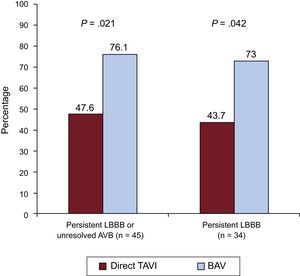
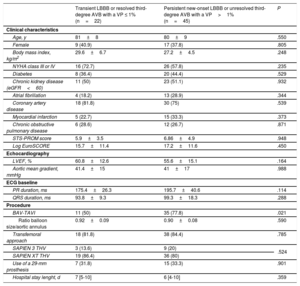
New-onset LBBB persistent at hospital discharge was observed in 57 (16.4%) patients, 16 (19.3%) in the direct TAVI group and 41 (15.5%) in the BAV group prior to valve implantation group (P=.45). In addition, 32 (9.2%) patients required PPM implantation after TAVI without differences between groups (direct TAVI: 9.6%, BAV prior to TAVI: 9.1%; P=.91) (Table 4). The reasons for PPM were third-degree AVB in 22 (68.7%) patients, new first-degree AVB and LBBB in 3 (9.4%) patients, second-degree AVB in 2 (6.2%) patients, intermittent LBBB and right bundle branch block in 2 (6.2%) patients, slow atrial flutter in 1 (3.1%) patient, and sinus node dysfunction with severe bradycardia in 2 (6.2%) patients. Overall, 77 (22.2%) patients had a persistent LBBB or third-degree AVB requiring PPM implantation without significant differences between groups (direct TAVI: 25.3%, BAV prior to TAVI: 21.2%; P=.434) (Table 4). There were 4 in-hospital deaths and 6 patients did not complete the 1 month follow-up visit. At 1-month follow-up, 45 patients had persistent LBBB or unresolved third-degree AVB with a ventricular pacing rate>1%, 10 (47.6%) in the direct TAVI group and 35 (76.1%) in the BAV prior to TAVI group, P=.021 (Figure 3). The main baseline clinical characteristics and echocardiographic and procedural findings of patients with new CA according to resolution of the CA at 1-month follow-up are shown in Table 5. These results did not change when differences between the BAV and direct TAVI groups (age, left ventricular ejection fraction, chronic obstructive pulmonary disease, transfemoral approach, and SAPIEN 3 device) were entered into a multivariate model. The electrocardiograms of patients with persistent CA tended to show a longer PR and QRS duration, but these differences were not statistically significant (175.4±26.3 vs 195.7±40.6; P=.114) and (93.8±9.3 vs 99.3±18.3; P=.288), respectively. The use of BAV prior to TAVI was the only factor associated with a lack of resolution of the CA (persistent LBBB or unresolved third-degree AVB with a ventricular pacing rate>1%) at 1-month follow-up (OR, 3.5; 95%CI, 1.17-10.43; P=.021).
Conduction Abnormalities Persistent at Hospital Discharge According to the Use of BAV During the TAVI Procedure
| Overall population (n=347) | Direct TAVI (n=83) | BAV-TAVI (n=264) | P | |
|---|---|---|---|---|
| LBBB | 57 (16.4) | 16 (19.3) | 41 (15.5) | .448 |
| PPM implantation | 32 (9.2) | 8 (9.6) | 24 (9.1) | .905 |
| LBBB or unresolved AVB requiring PPM | 77 (22.2) | 21 (25.3) | 56 (21.2) | .434 |
AVB, atrioventricular block, BAV, balloon aortic valvuloplasty; LBBB, left bundle branch block, PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
Persistent conduction abnormalities at 1-month follow-up according to the use of BAV prior to valve implantation. Rates of persistent LBBB or unresolved AVB with a ventricular pacing>1% and persistent LBBB at 1-month follow-up in patients undergoing BAV before valve implantation vs direct TAVI. AVB, atrioventricular block; BAV, balloon aortic valvuloplasty; LBBB, left bundle branch block; TAVI, transcatheter aortic valve implantation.
Baseline Characteristics and Procedural Findings of Patients With New-onset Conduction Abnormalities, According to the Persistence of Conduction Abnormalities at 1-month Follow-up
| Transient LBBB or resolved third-degree AVB with a VP ≤ 1% (n=22) | Persistent new-onset LBBB or unresolved third-degree AVB with a VP>1% (n=45) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 81±8 | 80±9 | .550 |
| Female | 9 (40.9) | 17 (37.8) | .805 |
| Body mass index, kg/m2 | 29.6±6.7 | 27.2±4.5 | .248 |
| NYHA class III or IV | 16 (72.7) | 26 (57.8) | .235 |
| Diabetes | 8 (36.4) | 20 (44.4) | .529 |
| Chronic kidney disease (eGFR<60) | 11 (50) | 23 (51.1) | .932 |
| Atrial fibrillation | 4 (18.2) | 13 (28.9) | .344 |
| Coronary artery disease | 18 (81.8) | 30 (75) | .539 |
| Myocardial infarction | 5 (22.7) | 15 (33.3) | .373 |
| Chronic obstructive pulmonary disease | 6 (28.6) | 12 (26.7) | .871 |
| STS-PROM score | 5.9±3.5 | 6.86±4.9 | .948 |
| Log EuroSCORE | 15.7±11.4 | 17.2±11.6 | .450 |
| Echocardiography | |||
| LVEF, % | 60.8±12.6 | 55.6±15.1 | .164 |
| Aortic mean gradient, mmHg | 41.4±15 | 41±17 | .988 |
| ECG baseline | |||
| PR duration, ms | 175.4±26.3 | 195.7±40.6 | .114 |
| QRS duration, ms | 93.8±9.3 | 99.3±18.3 | .288 |
| Procedure | |||
| BAV-TAVI | 11 (50) | 35 (77.8) | .021 |
| Ratio balloon size/aortic annulus | 0.92±0.09 | 0.90±0.08 | .590 |
| Transfemoral approach | 18 (81.8) | 38 (84.4) | .785 |
| SAPIEN 3 THV | 3 (13.6) | 9 (20) | .524 |
| SAPIEN XT THV | 19 (86.4) | 36 (80) | |
| Use of a 29-mm prosthesis | 7 (31.8) | 15 (33.3) | .901 |
| Hospital stay lenght, d | 7 [5-10] | 6 [4-10] | .359 |
AVB, atrioventricular block; BAV-TAVI, balloon aortic valvuloplasty during transcatheter aortic valve implantation; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; THV, transcatheter heart valve; VP, ventricular pacing.
Values are expressed as no. (%), mean±standard deviation or median [interquartile range].
At 1-month follow-up, LBBB persisted in 34 (64.1%) patients discharged with a new-onset LBBB: 7 (43.7%) patients in the direct TAVI group and 27 (73%) patients in the BAV prior to TAVI group, P=.042 (Figure 3).
DISCUSSIONIn patients undergoing TAVI with a balloon-expandable valve, more than half of CA (new-onset LBBB or advanced AVB) occurred before valve implantation. Conduction abnormalities, particularly new-onset LBBB, occurring before valve implantation tended to persist more frequently at discharge and at 1-month follow-up. The use of BAV during TAVI had no impact on the rate of persistent LBBB or unresolved advanced AVB at hospital discharge. However, BAV was associated with a higher rate of CA (LBBB or advanced AVB) persistence at 1-month follow-up.
As is the case with surgical aortic valve replacement,17 mechanical injury of the conduction system is considered the main mechanism underlying the occurrence of CA after TAVI.18 This is supported by pathological studies showing traumatic lesions with hemorrhage and necrosis at the level of the conduction system in patients with persistent CA post-TAVI.19,20 Transcatheter aortic valve implantation with a balloon-expandable valve involves the greatest force being exerted during actual valve deployment or later by the prosthesis itself. As a result, most CA could be expected to occur at the time of or immediately after valve implantation. In contrast, more than 50% of CA occurred before valve implantation in our study, and this finding is consistent with prior observations using self-expandable devices.4 The fact that direct TAVI (avoiding BAV) was not associated with a reduction in the overall rate of CA post-TAVI suggests that rather than BAV per se, the onset of CA before valve implantation identifies those patients with a more vulnerable conduction system, posing greater risk in these patients for new CA during valve manipulation and/or following valve implantation, irrespective of BAV. Variations in the anatomical relationship between differing components of the conduction system or a pre-existing subclinical disease, both identified as potential predictors of CA post-TAVI,3,5,21 may be responsible for this heightened vulnerability.
In addition, the rate of new CA following isolated BAV therapy is<10% in most studies,22,23 much lower than the 44% rate observed in this study or the 46% rate in the study by Nuis et al.,4 regardless of whether moderate BAV was performed during TAVI. In fact, the balloon size-to-aortic annulus ratio, which predicts the occurrence of CA after balloon valvuloplasty,22 was lower in this study compared with that reported when BAV is used as a definitive therapy in patients with critical aortic stenosis (0.91 vs>1.5).22 Considering the similarities in patient profiles, these differences in the rate of new CA attributable to BAV may be due to a high percentage of transient disorders which may resolve before the end of the procedure unless there is a second mechanical force (ie, subsequent valve implantation). Of note, the rate of transient CA during BAV procedures remains unknown.
The increased vulnerability of the conduction system to any trauma, even that of BAV, might have contributed to both the greater CA persistence at hospital discharge and the lower resolution of LBBB at 1-month follow-up when the CA occurred before valve implantation. In addition, and in accordance with prior studies,24 up to 77% of the LBBB that occurred during or immediately after valve implantation had resolved at hospital discharge, and 41% disappeared in less than 1 hour. Initial inflammation or edema, which has been touted as a cause of a transient CA following the first mechanical stress,25 may eventuate into a definitive injury when a greater mechanical stress is subsequently exerted. These observations are consistent with the results of Nuis et al.4 showing a higher rate of CA persistence when occurring before valve implantation. Different intrinsic characteristics of various prostheses might explain the different rates and clinical course of CA between balloon- and self-expanding devices.24 and the persistent force exerted by the nitinol of self-expanding devices may prevent the resolution of conduction disturbances.26
In the light of these results, the occurrence of CA before valve implantation may allow us to identify patients at higher risk of persistent (vs transient) CA. Therefore, a better knowledge of the timing of CA during TAVI might help us to anticipate the need for longer electrocardiogram monitoring or the need for PPM post-TAVI. The impact of post-TAVI LBBB on TAVI outcomes remains a topic of debate, and it has been suggested that these patients should undergo longer periods of electrocardiogram monitoring after TAVI due to the potential for progression to advanced AVB.1 However, new-onset LBBB occurring during or immediately after balloon-expandable valve implantation seems transient in most cases, and therefore, these patients may not require such long periods of electrocardiogram monitoring.1 In fact, more than three quarters of these LBBBs had disappeared before hospital discharge. However, it remains unknown whether the timing of CA and the use of BAV might have had an impact on the progression to AVB in patients with LBBB.
Likewise, this study suggests that the use of BAV during the TAVI procedure might be associated with a higher rate of unresolved AVB. Previous studies have suggested that up to 40% of patients receiving a PPM after TAVI had no need for ventricular pacing at follow-up.27,28 However, recommendations regarding the indication of PPM after TAVI cannot be concluded from the results of this study, considering that the absence of pacing dependency does not exclude the occurrence of paroxysmal advanced AVB.
LimitationsThe main limitation of this study is its small sample size. However, this was due in part to the exclusion of patients with pre-existing CA. In addition, this study currently represents the largest study with continuous electrocardiogram monitoring and systematic electrocardiogram recording during the TAVI procedure. The choice of direct TAVI or BAV preceding TAVI was not randomized, and we cannot exclude the influence of potential confounders on the results obtained. Two different generations of balloon-expandable valves (SAPIEN XT and SAPIEN 3), which might have a different impact on CA, were used. Although the direct TAVI strategy was more frequently used in patients receiving a SAPIEN 3 valve, the groups of persistent CA were well-balanced with respect to this variable.
CONCLUSIONSIn conclusion, more than half of CA following TAVI with a balloon-expandable valve occurred before valve implantation. Overall, CA occurring before valve implantation resolved less frequently at hospital discharge and at 1-month follow-up. Avoiding the use of BAV was not associated with a reduction in the overall rate of CA, but reduced the rate of CA persistence over time. These results improve our understanding of the mechanisms of CA during TAVI procedures and may help us to better determine the need for prolonged electrocardiogram monitoring after TAVI. Further studies are warranted to determine the potential benefits of avoiding BAV prior to TAVI.
CONFLICTS OF INTERESTA. Regueiro and M. Del Trigo are supported by a grant from the Fundación Alfonso Martín Escudero (Madrid/Spain). R. Puri is partially supported by a grant of the Research Center from the Quebec Heart and Lung Institute (Quebec/Canada). J. Rodés-Cabau has received research grants from Edwards Lifesciences and Medtronic. V. Auffret received fellowship support from the Fédération Française de Cardiologie and research grants from Abbott, Edwards Lifesciences, Medtronic, Biosensors, Terumo and Boston Scientific. C. Chamandi received fellowship support from Edwards Lifesciences.
- –
The continual development of TAVI has significantly reduced procedural complications. Nevertheless, CA remain frequent and their incidence is unlikely to decrease in the near future. In fact, an increased rate of CA has been reported with some of the newer generation transcatheter valves. Predictive factors of CA for both balloon- and self-expanding transcatheter prostheses have been elucidated but little is known about the timing and mechanisms underlying the occurrence of conduction disturbances during TAVI.
- –
This is the first study evaluating the timing of onset and resolution of CA in patients undergoing TAVI with a balloon-expandable valve and determining the impact of BAV on the persistence of CA post-TAVI.
- –
The main results of the study showed that more than half of CA following TAVI with a balloon-expandable valve occurred before valve implantation. Overall, CA–particularly LBBB–occurring before valve implantation resolved less frequently at hospital discharge and at 1-month follow-up. Avoiding the use of balloon predilation reduced the rate of CA persistence over time.
.
This study will be included in a Medicine PhD thesis at the Autonomous University of Barcelona.