We have read with interest the study by Riziq-Yousef Abumuaileq et al.1 in which they demonstrate a similar predictive capacity for the HAS-BLED, ORBIT and ATRIA scoring systems for predicting bleeding complications in patients with atrial fibrillation (AF) being treated with vitamin K antagonists. However, the study that initially validated the ORBIT scoring system2 showed it to be superior to the other 2 scoring systems. Based on these seemingly discordant results, and taking into account the increasing use of direct oral anticoagulants (DOACs), with its specific characteristics in our area, we consider the validation and comparison of these scoring systems to be clinically significant in patients in our environment starting treatment with DOACs.
To this end, a retrospective study was conducted in 3 Spanish hospitals. Between January 2013 and December 2014, 973 consecutive patients with nonvalvular AF who started treatment with DOACs were included. Patients with an indication of temporary anticoagulation or different from AF and those with hypertrophic cardiomyopathy, moderate/severe rheumatic mitral stenosis, carriers of mechanical valvular prostheses or those already taking DOACs were excluded. The 3 bleeding risk scores (HAS-BLED, ATRIA and ORBIT) were calculated in 970 patients (99.7%). During the follow-up period (mean, 646 [470-839] days), bleeding complications were collected by reviewing electronic medical records and through telephone calls in 99.8% of the patients. Bleeding complications were classified according to the International Society on Thrombosis and Hemostasis criteria.3,4
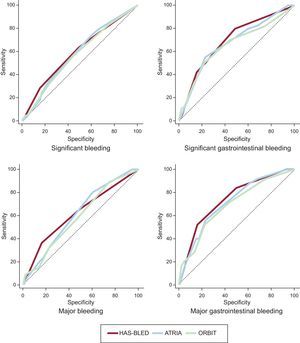
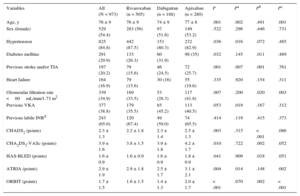
The population characteristics are shown in Table. A total of 505 patients (51.9%) received rivaroxaban; 188 (19.3%) received dabigatran and 280 (28.8%) received apixaban. During the follow-up period, there were 101 clinically significant bleeding episodes (6.11/100 people/y), 47 major bleeding episodes (2.76/100 people/y), 40 significant gastrointestinal bleeding episodes (2.33/100 people/y), 25 major gastrointestinal bleeding episodes (1.46/100 people/y), 5 episodes of intracranial bleeding (0.29/100 people/y) and 102 deaths (5.85/100 people/y), 34 of which were of cardiovascular origin (1.95/100 people/y). The bleeding rate increased in line with the risk scores (). All the risk scores showed moderate discriminatory ability (Figure) for both major bleeding—HAS-BLED, 0.62 (95% confidence interval [95%CI], 0.59-0.65); ATRIA, 0.61 (95%CI, 0.58-0.64) and ORBIT, 0.59 (95%CI, 0.56-0.62)—and significant bleeding—HAS-BLED, 0.59 (95%CI, 0.56-0.62); ATRIA, 0.58 (95%CI, 0.55-0.61) and ORBIT, 0.57 (95%CI, 0.54-0.60). The discriminatory ability was somewhat higher for gastrointestinal bleeding—major gastrointestinal bleeding: HAS-BLED, 0.74 (95%CI, 0.71-0.76); ATRIA, 0.71 (95%CI, 0.68-0.74) and ORBIT, 0.69 (95%CI, 0.66-0.72); significant gastrointestinal bleeding: HAS-BLED, 0.69 (95%CI, 0.66-0.72); ATRIA, 0.67 (95%CI, 0.64-0.70) and ORBIT, 0.65 (95%CI, 0.62-0.69). Comparison of the ROC curves of the bleeding risk scoring systems showed no significant differences in any type of event in the general population or after stratification by type of DOAC (all P > .05) ().
Baseline Population Characteristics
| Variables | All (N = 973) | Rivaroxaban (n = 505) | Dabigatran (n = 188) | Apixaban (n = 280) | P | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|---|
| Age, y | 76 ± 9 | 76 ± 9 | 74 ± 9 | 77 ± 8 | .001 | .002 | .491 | .001 |
| Sex (female) | 529 (54.4) | 283 (56) | 97 (51.6) | 149 (53.2) | .522 | .296 | .446 | .731 |
| Hypertension | 825 (84.8) | 442 (87.5) | 151 (80.3) | 232 (82.9) | .036 | .016 | .072 | .485 |
| Diabetes mellitus | 291 (29.9) | 133 (26.3) | 60 (31.9) | 98 (35) | .032 | .145 | .011 | .489 |
| Previous stroke and/or TIA | 197 (20.2) | 79 (15.6) | 46 (24.5) | 72 (25.7) | .001 | .007 | .001 | .761 |
| Heart failure | 164 (16.9) | 79 (15.6) | 30 (16) | 55 (19.6) | .335 | .920 | .154 | .311 |
| Glomerular filtration rate <60mL/min/1.73 m2 | 339 (34.9) | 169 (33.5) | 53 (28.3) | 117 (41.8) | .007 | .200 | .020 | .003 |
| Previous VKA | 377 (38.8) | 179 (35.5) | 85 (45.2) | 113 (40.5) | .053 | .019 | .167 | .312 |
| Previous labile INRd | 243 (65.0) | 120 (67.4) | 49 (59.0) | 74 (65.5) | .414 | .119 | .415 | .373 |
| CHADS2 (points) | 2.3 ± 1.3 | 2.2 ± 1.8 | 2.3 ± 1.4 | 2.5 ± 1.3 | .003 | .315 | < .001 | .086 |
| CHA2DS2-VASc (points) | 3.9 ± 1.6 | 3.8 ± 1.5 | 3.9 ± 1.8 | 4.2 ± 1.7 | .010 | .722 | .002 | .052 |
| HAS-BLED (points) | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 | 1.8 ± 0.9 | .041 | .909 | .018 | .051 |
| ATRIA (points) | 2.9 ± 1.9 | 2.9 ± 1.8 | 2.5 ± 1.7 | 3.1 ± 2.1 | .004 | .014 | .148 | .002 |
| ORBIT (points) | 1.7 ± 1.5 | 1.6 ± 1.5 | 1.4 ± 1.3 | 2.0 ± 1.7 | < .001 | .070 | .002 | < .001 |
INR, international normalised ratio; TIA, transient ischaemic attack; VKA, vitamin K antagonists.
Values are expressed as mean ± standard deviation and No. (%).
The results of this study demonstrate that the 3 scoring systems assessed show moderate capacity with no significant differences in discriminating bleeding in patients with nonvalvular AF starting treatment with DOACs. Our results confirm the findings of Riziq-Yousef Abumuaileq et al.1 and also extend their use to patients with AF being treated with DOACs. Given the increasing use of these anticoagulant agents in routine clinical practice, we consider our findings to be clinically significant. Bleeding risk scoring systems are highly useful for identifying patients with a high risk of bleeding who would probably benefit from closer monitoring. Among the different scoring systems available, the HAS-BLED scoring system has become a benchmark in routine clinical practice, as it has various advantages over other published scoring systems. Because this scoring system has been previously validated in various populations with various antithrombotic regimens, it can be applied to a wide population group. Furthermore, the presence of reversible bleeding risk factors that can be modified by clinicians allows patients’ bleeding risk treatment to be considered a nonstatic process, unlike other scoring systems that do not include these potentially modifiable factors. The European Society of Cardiology recently published new clinical practice guidelines for the management of AF.5 Unlike previous guidelines, these do not recommend using a particular bleeding risk scoring system, but rather focus on using any of them to identify and correct potentially modifiable bleeding risk factors.