In recent years, three-dimensional (3D) printed models have been incorporated into cardiology because of their potential usefulness in enhancing understanding of congenital heart disease, surgical planning, and simulation of structural percutaneous interventions. This review provides an introduction to 3D printing technology and identifies the elements needed to construct a 3D model: the types of imaging modalities that can be used, their minimum quality requirements, and the kinds of 3D printers available. The review also assesses the usefulness of 3D printed models in medical education, specialist physician training, and patient communication. We also review the most recent applications of 3D models in surgical planning and simulation of percutaneous structural heart interventions. Finally, the current limitations of 3D printing and its future directions are discussed to explore potential new applications in this exciting medical field.
Keywords
The diagnosis and treatment of structural and congenital heart disease has traditionally relied on the analysis of images obtained by echocardiography, angiography, computed tomography (CT), and magnetic resonance imaging (MRI). To fully appreciate the complexity of the conditions they treat, clinical cardiologists, cardiac catheterization specialists, and surgeons depend on skills, acquired through years of experience, in mentally assembling the anatomical topology of the heart from these 2-dimensional images, as if they were solving a 3-dimensional (3D) puzzle. To make their task even more challenging, these images are usually reproduced as enlarged projections on a flat screen and thus do not represent structures at their real size. Virtual 3D projections offer some appreciation of topology, but these images cannot be held and give an imprecise impression of structural depth and the proximity of structures in distinct spatial planes.
Resolving these limitations has recently become a realistic prospect with the advent of 3D printing, a technology originating in engineering and the aeronautical industry that has begun to find applications in the world of medicine. The aim of this review is to introduce readers to 3D printing technology and to provide an overview of the imaging requirements for generating a 3D model. We review current scientific evidence on the usefulnessof 3D cardiovascular models for medical education, patient communication, specialist physician training, and the applications of 3D printing in cardiovascular surgery and percutaneous structural heart interventions.
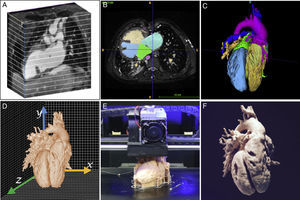
INTRODUCTION TO THE TECHNOLOGYGenerating a 3D model is a complex process requiring a multidisciplinary team of radiologists, cardiologists, pediatricians, and engineers. These specialists need to work together on each of the following steps: medical image acquisition, segmentation, computer-assisted design, and finally 3D printing (Figure 1).
Medical images suitable for 3D printing must be isotropic, of high spatial resolution in order to provide rich detail, and of sufficiently high contrast to distinguish between adjacent structures. High resolution and contrast minimizes processing time and ensures an exact anatomical replica. Low resolution images or the presence of artifacts can give the appearance of continuity between neighboring structures such as the aorta and pulmonary artery; when transferred to the 3D model, these characteristics will produce a false diagnosis of an aortopulmonary window.
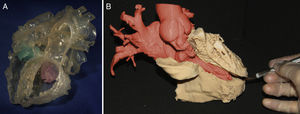
Each imaging methodology has its advantages and limitations. For printing 3D images of large vessels, heart chambers, and ventricular septal defects, the best options are CT1–8 and MRI.9–15 The desired isotropic resolution is 0.5 to 1.25mm3 for CT and 1.5 to 1.8mm3 for MRI. In contrast, echocardiography is the preferred imaging technique for 3D printing of heart valves, the subvalvular apparatus, and the interatrial septum.16–21 As with clinical practice, multimodal evaluation is applicable to 3D printing, yielding 3D models with a high level of detail (Figure 2).16 Another key factor in the choice of imaging modality for 3D printing is the center's level of expertise in different imaging modalities.
A: Hybrid 3-dimensional (3D) model created from computed tomography and 3D echocardiography images to obtain the blood volume and the tricuspid and mitral valves (green and pink, respectively). Reproduced with permission from Gosnell et al.16 B: 3D model representing the left ventricular myocardium (ochre) and the right atrium and aorta (red).
The next step is segmentation, the delineation of the cardiovascular structures of interest and exclusion of irrelevant noncardiac structures such as bone and lung. There are 2 basic segmentation strategies for heart modeling: blood pool segmentation to derive the chamber-wall structure (Figure 2A), or myocardium segmentation (Figure 2B). Models derived from blood pool segmenation provide a rapid understanding of the disease,22 structural dimensions, the coronary arteries, and the great vessels; in contrast, 3D models of the myocardium are ideal for delineating ventricular septal defects and specimen dissection and therefore allow simulation of surgical strategies.13 Available software for segmenation includes comprehensive commercial packages (Materialise, Leuven, Belgium) and freeware (ITK snap23), the latter being an attractive option for small centers taking their first steps in this technology. The various segmentation algorithms and methodologies are covered in depth in Suárez-Mejías et al.24 and Byrne et al.25
Three-dimensional PrintingThree-dimensional printing is a form of additive manufacturing in which the 3D object is contructed by the layer-on-layer addition of new material to an existing surface. The most common printing methods are fused deposition modeling, selective laser sintering, and stereolithography. Other much more complex 3D printing methods used to print living tissues fall outside the scope of this review, but are described in detail elsewhere.26,27
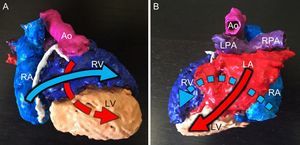
APPLICATIONS OF 3-DIMENSIONAL PRINTINGMedical EducationMedical education is subject not only to economic limitations, but also to ethical, legal, and cultural concerns that restrict the availability of cadavers. Modern technology and 3D printing offer possible solutions in this area and have been used to model human anatomy in a variety of settings.28 This application of 3D printing is especially relevant to the teaching of congenital heart disease because it can expose students to the full spectrum of malformations and the variability that may exist even within a single condition. Handling 3D models engages students’ sight and touch to achieve rapid understanding of anatomical defects, including complex phenomena such as criss-cross atrioventricular connections (Figure 3). 3D models have been used to teach the morphological characteristics of tetralogy of Fallot. Classes that used the 3D models were more positively evaluated by students than those that did not, which was reflected in higher student satisfaction scores and higher retention rates.29
Three-dimensional (3D) model of a heart with criss-cross atrioventricular connections. A: Anterior view. B: Posterior view. The model shows the contralateral positions of the right atrium (RA) and right ventricle (RV) and of the left atrium (LA) and left ventricle (LV), with the associated crossing of the inflow tracts (arrows). Ao, aorta; LPA, left pulmonary artery; RPA, right pulmonary artery.
Moreover, the interconnectivity provided by the internet makes it easy to share digital files through online networks, allowing users to rapidly access and print 3D models of the disease of interest. Making 3D images available through online collections can provide hospitals and research centers with free access to a broad spectrum of heart conditions.30
Even more importantly, 3D models promise to transform teaching in ways that go beyond the lecture hall, and over the next few years are set to revolutionize medical training, especially in percutaneous interventions.31 Acquiring the necessary skills in these procedures requires a major dedication of financial and human resources, is time consuming, and puts patients at risk. These concerns are behind current moves to make simulations a required element of cardiac catheterization training, part of a joint effort affecting the clinical practice guidelines of several international societies, including the Cardiovascular and Interventional Radiological Society of Europe, the Society for Cardiovascular Angiography and Interventions, the Society of Interventional Radiology, and the Radiological Society of North America.31,32 Simulations involving 3D cardiac models allow trainees to practice with a diverse range of scenarios, repeat technical procedures, and learn from mistakes without putting patients at risk.10 Simulations can be used to train specialists before they carry out their first procedures on patients, for training reinforcement, and for instruction in new and more complex procedures.
Another application of 3D models is as a tool for explaining procedures to patients during medical consultations, a practice demonstrated to improve patient satisfaction and instil a sense of involvement with medical staff.11
Planning Heart SurgeryCongenital Heart DiseasePrinted 3D models have demonstrated their usefulness in revealing the target anatomy before intervention in a wide range of congenital heart conditions. This gives specialists a level of confidence not achieved with virtual 3D imaging techniques. For example, the use of printed 3D models in presurgery planning can improve postsurgery blood flow after complex surgical repairs such as the Nikaidoh procedure in patients with transposition of the great arteries associated with interventricular communication and outflow tract obstruction.12
Printed 3D models have also been used to demonstrate suitability for biventricular repair among patients who would otherwise have been palliated to univentricular repair, as demonstrated by Farooqi et al. in a patient with a double outlet right ventricle and a remote ventricular septal defect.9
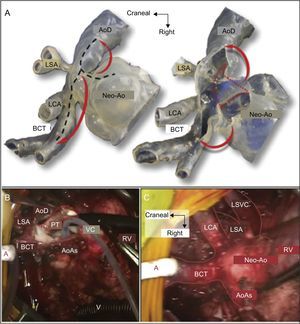
Another highly attractive application of 3D models is in planning interventions to treat left heart hypoplasia, including the Glenn, Norwood, and Fontan procedures.2,8,33 For example, Kiraly et al. used a 3D model to plan the Glenn procedure in a 5-year-old boy with left heart hypoplasia and aortic obstruction at several locations (Figure 4).2 Finally, 3D models can also be a useful pretransplant planning aid for intervention in patients with a failing Fontan circulation, in whom the distortions produced by previous surgery can necessitate technical approaches different from those used in conventional patients.34
A: Glenn procedure simulation with a 3-dimensional (3D) model simulating the surgeon's intraoperative view. The discontinuous black lines mark the desired incision. The 3D model was opened and the stenotic segments resected (discontinuous red lines). The autologous tissue leaflets were folded to enlarge the aortic arch (red arrows). B: Intraoperative view during surgery. The incision was made from the neoaorta to the junction with the transverse arch and was then extended toward the common brachiocephalic trunk and the descending aorta. The stenotic posterior membrane was resected (discontinuous red line). The cardioplegic solution was injected into the native aortic root through a 6 Fr-Foley catheter. C: Intraoperative view after completion of the autologous patch enlargement. AoAs, native ascending aorta; AoD, descending aorta; BCT, brachiocephalic trunk; LCA, left carotid artery; LSA, left subclavian artery; LSVC, left superior vena cava; Neo-Ao, neoaorta (native pulmonary trunk); PT, pulmonary trunk; RV, right ventricle; VC, venous cannula. Adapted with permission from Kiraly et al.2
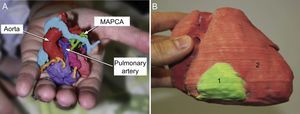
Printed 3D models are thus finding applications in the evaluation of internal cardiac features such as defects in the atria13,21 and ventricles20,33,35; however, their use is not limited to the heart, and they have also been used to evaluate vascular anatomy, for example aortopulmonary collaterals in patients with pulmonary atresia and ventricular septal defects. Printed 3D models provide highly detailed information about the position and intricate path of the collaterals, which is extremely useful in presurgery planning (Figure 5A).4,8
A: Actual-size 3-dimensional (3D) model of the heart and great vessels of a 1-day-old male patient with pulmonary atresia, confluent pulmonary branches connected to the pulmonary artery, and multiple aortopulmonary collateral arteries (MAPCA). Reproduced with permission from Ryan et al.4 B: 3D model of a cardiac tumor (1, green) located in the free wall of the right ventricle (2, red). Reproduced with permission from Schmauss et al.35
For patients with cardiac tumors or hypertrophic cardiomyopathy, the key factor in selecting the surgical strategy is lesion size, which determines the choice between partial or total resection and heart transplant. In these patients, 3D printing with different materials and colors is especially useful because it can reveal lesion position, the tumor vasculature, and the spatial relationship between the lesion and the valves, subvalvular apparatus, and papillary muscles. This application has already been used to plan the total resection of an right ventricular (RV) fibroma35 and septal myectomy in a patient with hypertrophic cardiomyopathy.5
Aortic DiseaseDue to the varied range of aneurysms, aortic dissections, and surgical histories, surgery of the aortic arch remains highly challenging. Through the use of 3D models, aortic diameters and anatomy can be reproduced accurately, allowing preoperative planning and in vitro simulation. The precision afforded by 3D modeling is essential for selecting an appropriate endoprosthesis, as described by Schmauss et al. for a patient with an extensively atherosclerotic aortic aneurysm considered for the frozen elephant trunk procedure.36 The same group also used 3D models to plan aortic valve replacement surgery in a patient with a history of surgical coronary revascularization.37 Another potential application of 3D models is in the design and testing of personalized external aortic root support implants for Marfan syndrome patients; these implants are an innovative alternative for stabilizing the distended aortic root and minimizing dissection risk.38
Simulating Percutaneous Structural Heart InterventionAs the range of structural heart disorders treatable by percutaneous catheter interventions has increased, so has the complexity of these procedures. Printed 3D models can play a key role here, both in interventional planning and in training cardiac catheterization specialists, as proposed in a recent special report.39 Just 1 year on from that report, new applications have emerged that greatly surpass the original expectations.
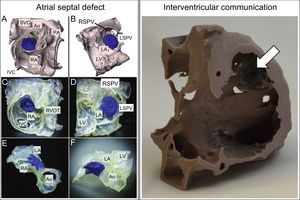
Septal Defect ClosureThe risk of an atrial septal defect closure device becoming dislodged after implantation is usually determined by the size of the defect and above all by the catheterization specialist's experience and skill in judging whether the defect rims are large and strong enough to support the device.40 It is sometimes difficult to determine if the risk of device embolization exceeds the risk of surgery; in this situation, 3D models can be a useful decision-making tool. This potential was highlighted in 2008 by Kim et al., who produced the first prototype printed 3D models to illustrate the broad spectrum of atrial septal defect morphology and size.41 The next step toward in vitro simulation came some years later in a study by Chaow et al., who demonstrated the utility of printed 3D models in selecting the appropriate device size for defects with an inadequate posteroinferior rim. Printed 3D models can also be useful when doubts remain about appropriate device localization after release, helping to exclude residual compression in neighboring structures (Figure 6, left).42
Left. Testing the percutaneous closure of a 16 mm ostium secundum interatrial communication with a 17 mm Amplatzer Septal Occluder (blue). A and B: Segmentation from computed tomography images. C-F: Assorted views of the 3-dimensional model to check for appropriate device positioning and compression of neigboring vessels. Reproduced with permission from Bartel et al.42 Right. Simulated closure of a postinfarction ventricular septal defect with a 20 mm Amplatzer Septal Occluder (arrow), viewed from the right atrium. Reproduced with permission from Lazkani et al.43 Ao, Aorta; IVC, inferior vena cava; LA, left atrium; LSPV, left superior pulmonary vein; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RSPV, right superior pulmonary vein; RVOT, right ventricular outflow tract; SVC, superior vena cava.
Printed 3D models have also assisted decision-making in relation to ventricular septal defects. For example, Lazkani et al. used in vitro simulation with 3D models to select the appropriate device type and size for a patient with a postinfarction ventricular septal defect, achieving a positive outcome with no residual shunt (Figure 6, right).43
Mitral Valve DiseaseThe variability and complexity of the mitral valve makes percutaneous treatment of mitral defects particularly challenging. In a previous article in Revista Española de Cardiología, Vaquerizo et al. described how 3D models can improve knowledge of local anatomy and potential interactions with transcatheter mitral valve replacement devices.44 The authors also highlighted the industrial potential of 3D mitral-valve modeling in the development and testing of new devices for a wide range of morphologies.
Another article described the use of a 3D model to plan percutaneous mitral annuloplasty.45 Simulation with the 3D model helped cardiac catheterization specialists to trial the complex navigational manouvers needed to advance catheters from the aorta to the left ventricle and position the catheter tip between the 2 papillary muscles and close to the mitral annulus. The 3D model was used to preprocedurally select the size of the deflectable guiding catheter and to optimize catheter positioning relative to the mitral annulus, resulting in a reported successful and safer outcome.
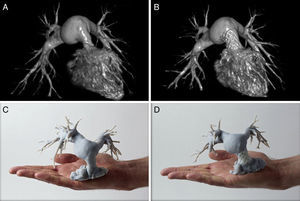
Pulmonary Valve ReplacementMost patients treated by transannular patch repair for tetralogy of Fallot are not indicated for transcatheter pulmonary valve replacement because of their large and irregular RV outflow tracts. In a landmark study, Phillips et al. used CT images to generate 3D models for 8 consecutive Fallot patients with a dilated RV outflow tract.46 The 3D models were used to develop individualized procedures to create a stable landing zone in a hybrid approach combining RV outflow tract remodeling with transcather pulmonary valve replacement. When the minimum right outflow tract diameter was > 26 mm, 1 or more covered stents were simultaneously implanted adjacent to a bare landing-zone stent onto which the pulmonary valve was then released. Finally, the lumen of the adjacent covered stent(s) was occluded with an Amplatzer Vascular Plug II (St. Jude Medical). In the authors’ estimation, the 3D models helped the whole multidisciplinary team to visualize the intervention, try out different strategies, and design individualized solutions for a population with widely differing cardiac anatomies. A similar approach has been used to treat transposition of the great arteries in a 15-year-old patient who had developed combined neo-pulmonary stenosis and regurgitation after arterial switch operation as a neonate (Figure 7).47
Virtual reconstructions (A and B) and 3-dimensional models (C and D) from a 15-year-old male patient with great artery transposition and supravalvular pulmonary stenosis. Reconstructions and models were created after previous arterial switch surgery (A and C) and after implantation of a Medtronic Melody valve (B and D). Reproduced with permission from Poterucha et al.47
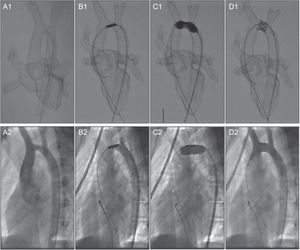
Endovascular stenting is an alternative to surgery in patients with aortic arch hypoplasia associated with aortic coarctation; however, an overlong stent can block the supra-aortic trunks, whereas a small stent can migrate from the implantation site.48 Our group used a 3D model derived from MRI scans to simulate intervention in a 15-year-old male patient with transverse aortic arch hypoplasia10 (Figure 8). Because the model was radioopaque, fluoroscopy guidance could be used to visualize navigation with conventional catheters. This allowed unlimited simulation of different interventional strategies to determine the optimal stent length and release site. We were then able to faithfully reproduce the selected procedure in the patient, using the same technique, catheters, and devices, thereby minimizing intraprocedural improvisation and patient risk and achieving a positive outcome.
Stent placement in a 15-year-old male patient with aortic arch hypoplasia. Comparison of preintervention simulation with a 3-dimensional printed model (A1-D1) and the real intervention in the patient (A2-D2). Reproduced with permission from Valverde et al.10
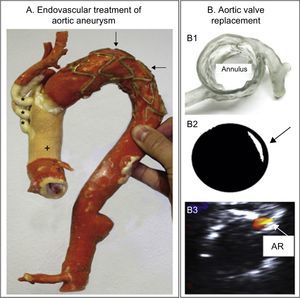
Printed 3D models have also been used to support endovascular repair of aortic aneurysms. Sodian et al. used 3D models to design bespoke devices to seal perianastomotic drains after ascending aorta and transverse aortic arch replacement surgery.49 The authors found not only that simulations with the 3D model were useful in selecting the best strategy for pseudoaneurysm closure, but also that they helped to engineer an individualized device tailored to the patient's anatomy. In another article, the same research team used this technology to plan transcatheter aortic valve replacement in a patient with severe aortic stenosis and a porcelain aorta (Figure 9A).36
A: 3-dimensional (3D) model after the frozen elephant trunk procedure to treat an extensively atherosclerotic aortic aneurysm. The image shows the prosthetic ascending aorta (+) and supraaortic trunks (*) and the aortic arch stenting (arrows). Reproduced with permission from Schmauss et al.36 B1: 3D model evaluating aortic root and coronary artery morphology. B2: Retrospective prediction of paravalvular aortic regurgitation (arrow) by shining light on a printed Edwards-SAPIEN valve positioned in the 3D model. B3: Transesophageal echocardiography in the patient showing residual aortic regurgitation (AR) after valve placement. Reproduced with permission from Ripley et al.50
A more systematic examination of the use of 3D models to predict the outcome of transcatheter aortic valve replacement was presented by Ripley et al. (Figure 9B).50 The study examined a series of 16 patients, 9 with paravalvular aortic regurgitation on postprocedure echocardiography and 7 with no residual leakage. The authors created 3D models for these patients from preprocedure cardiac CT scans and used these models to retrospectively predict the shape and location of the periprosthetic leak. The 3D models accurately predicted dehiscence in 6 of the 9 patients with paravalvular aortic regurgitation (6 true positives and 3 false negatives) and excluded residual leakage in 5 of the 7 control patients (5 true negatives and 2 false positives). The authors recognized that major challenges remain, such as ensuring that models accurately reproduce the mechanical properties of tissues; however, they concluded that this technology is making giant steps toward clinical implementation in the not too distant future.
Vascular ObstructionPulmonary venous baffle obstruction is a well-known complication in patients with transposition of the great arteries corrected by the Senning technique. Catheter approaches are a viable alternative to surgery but require meticulous planning in order to avoid complications. Olivieri et al. created a 3D model to plan catheter access and evaluate the positioning of several stents in the stenotic baffle and their relationship to neighboring structures.7 The authors found that the in vitro simulation improved procedure efficiency in the patient and reduced radiation dose, procedure time, and potential complications.
LIMITATIONSMathur et al.51 recently warned that, like almost all new technologies, 3D printing is no panacea, and that physicians should be wary of getting carried away by their initial enthusiasm. Instead, physicians should evaluate 3D printing critically, meticulously scrutinizing its limitations in order to identify ways to overcome them.
A recent meta-analysis of 158 studies found that the most prominent limiting factors in 3D printing were precision, preparation time, and cost.28 Probably one of the most important determinants of precision is segmentation. Delimiting cardiac tissues currently requires manual and subjective intervention by a clinician experienced in image analysis. This operator-dependent process inevitably translates into intraobserver and, above all, interobserver variability. This concern is driving efforts to develop programs able to achieve 100% automatic segmentation; however, a recent study by Cai et al. demonstrated that there is still a long way to go due to variability among the different algorithms used.52 Notably, the reproducibility of 3D models is not being tested by comparison with radiological images; instead, current efforts to definitively validate 3D modeling approaches are based on comparison with the real anatomy of autopsy samples.53,54 Analyzing the variables affecting the precision of 3D printing thus appears to be difficult and complicated. Nonetheless, such an analysis is essential to overcome the current lack of quality assurance; moreover, medical regulatory agencies will doubtless soon seek to establish quality standards before printed 3D models become a standard part of clinical practice.
Another major limiting factor is the time needed to create 3D modelsis. However, it is important to balance the time invested in creating 3D models against benefits in other areas. For example, 10 minutes of surgery time saved due to appropriate planning with a 3D model can represent an economic saving equivalent to 1 hour of work at the computer,55 and this is without considering less tangible benefits such as patient safety. Final-cost calculations for a 3D model should include all the links in the manufacturing chain, including clinical staff, engineers, software, and the printer and materials. Our group's approach is to create 3D models with a sufficient level of precision to allow diagnosis while keeping costs as low as possible. Keeping costs down is essential if 3D printing technology is to be taken up widely and not remain the restricted domain of private hospitals or those with a high patient volume. With freeware and cheaper printers, the cost of a 3D model is approximately €500, compared with €1000 for a commercially sourced model or one obtained with a faster printer or with a higher quality finish.
A final limitation to consider is that most studies to date have examined a single patient series, and there have as yet been no multicenter, randomized, large-scale studies that could demonstrate the true impact of this technology on both surgery and percutaneous intervention.
FUTURE DIRECTIONSAs segmentation becomes ever more automated, and as faster and cheaper 3D printers enter the market, more hospitals and universities will dedicate funds to printing their own models, which will become a standard complement to conventional radiological imaging studies. This will require multidisciplinary collaboration in the broadest sense, involving radiologists, clinical cardiologists, cardiac catheterization specialists, and surgeons, and above all incorporating engineers into clinical teams.
There is no doubt that 3D printing is an immensely promising technology, set to have an enormous impact on medicine generally and on the treatment of congenital and structural heart diseases in particular. Widespread incorporation of 3D printing will be a major advantage in tackling new diagnostic and therapeutic challenges.
FUNDINGPart of the research presented here was funded by the Instituto de Salud Carlos III, an arm of the Ministry of Science and Innovation, Proyecto de Investigación en Salud, Fondo de Investigación Sanitaria number PI14/00180.
CONFLICTS OF INTERESTNone declared.
We thank Saúl Valverde for his invaluable review of this manuscript and G. Gómez, M.N. Velasco, C. Suárez-Mejías, C. Escabias, and J.A. Rivas for their contribution to research into 3D models.