The undeniable advances in the field of oncology have finally led to a decrease in overall cancer-related mortality. However, this population of long-term cancer survivors is now facing a shift toward a substantial increase in cardiovascular morbidity and mortality. Because the development of overt cardiotoxicity can be associated with poor outcomes, preclinical identification of cardiac toxicity is important. This will promote early instauration of treatments to prevent overt heart dysfunction and allow oncologists to continue cancer therapy in an uninterrupted manner. Surveillance strategies for the early detection of cardiac injury include cardiac imaging and biomarkers during treatment. In this review, we outline existing cardiac imaging modalities to detect myocardial changes in patients undergoing cancer treatment and in survivors, and their strengths and limitations.
Keywords
Advances in the early detection and treatment of malignancies have resulted in a 20% decline in cancer mortality.1 However, cardiovascular disease (CVD) has become an important competing risk for morbidity and mortality in cancer survivors.2 For example, in breast cancer survivors older than 66 years who survived more than 5 years, CVD exceeds breast cancer as the leading cause of death.2 This heighted risk of CVD is due to a combination of shared risk factors for cancer and CVD, the direct impact of cancer therapy on the cardiovascular system, and the gap in the cardiac care of patients with cancer.3–5 Optimization of preexisting conditions before treatment is important, but is unlikely to be sufficient as a sole strategy to prevent CVD. Particularly for the prevention of cancer therapy-related heart failure (HF), strategies to identify early myocardial injury are needed so that targeted therapy can be instituted to prevent overt HF. Cardiac imaging and serum biomarkers have been demonstrated to be key strategies in identifying early myocardial injury. Serum biomarkers have shown tremendous promise but have some limitations, including the lack of clarity on the best biomarker to use, the timing of measurements, and the thresholds to define abnormality.6 There has been increasing enthusiasm for the use of cardiac imaging to detect cardiac injury as it provides direct assessment of myocardial function.7 In this review, we outline existing cardiac imaging modalities to detect myocardial changes in patients undergoing cancer treatment and in survivors and their strengths and limitations. We also provide some practical suggestions for clinicians involved in the cardiac care of patients with cancer.
3D ECHOCARDIOGRAPHYControversies in the Definition of CardiotoxicityDifferent definitions of cardiotoxicity have been used historically with practical implications for how patients are managed.8 The strongest controversy concerns the definition of cancer therapy-related cardiac dysfunction (CTRCD) both in clinical trials and consensus documents.3,9 In the modern era, overt clinical HF and cardiac death occur in 5% to 6% of treated patients.10,11 Asymptomatic deterioration in left ventricular ejection fraction (LVEF), associated with a higher incidence of symptomatic HF is documented in as many as 20% of patients depending on the cancer treatment.12–14
All definitions of CTRCD are based on a serial decline in LVEF. Unfortunately, there are no universal threshold changes to define CTRCD. The American Society of Echocardiography Consensus document defines CTRCD as a LVEF drop ≥ 10% to a value of < 53%,15 based on new American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommendations for chamber quantification.16 These recommendations have been endorsed by the Canadian Cardiovascular Consensus Statement.17 Recently, the European Society of Cardiology 2016 position paper considers the lower limit of normal LVEF by echocardiography as 50%, in line with the definition commonly used in registries and trials in patients with cancer.18
Independent of normal reference ranges, relative changes between baseline and follow-up LVEF are needed to appropriately identified CTRCD. If LVEF decreases > 10% to a value below the lower limit of normal, angiotensin-converting enzyme inhibitors (or angiotensin receptor blockers) in combination with beta-blockers are recommended (unless contraindicated) to prevent further left ventricular (LV) dysfunction or symptomatic HF.15,17,18 If LVEF decreases > 10%, to a value that does not drop below the lower limit of normal, patients should undergo repeat assessment of LVEF promptly. In addition, these patients should be considered for advance echo imaging monitoring to avoid delays in HF therapy initiation.15,17,18
This article focuses on the ASE definition of CTRCD (lower limit of normal considered as an LVEF < 53%) to improve the early detection and prompt therapy of cardiotoxicity, which are crucial for substantial recovery of cardiac function.19,20 In fact, Wang et al.11 demonstrated that even normal LVEF within 5 points of the lower limits of normal was associated with a near-to-3 fold increase in the rate of cardiac events in patient treated with anthracyclines
We Need Precise LVEF Measurements: Pros and Cons of 3DE-LVEFIn cancer patients, serial evaluation of LVEF must be reliable enough to identify true changes in ventricular function leading to subsequent clinical and therapeutic decisions.21 While the imaging modality for monitoring should be based on local institutional expertise, 2-dimensional echocardiography (2DE) is increasingly used due to its widespread availability and safety. This modality allows for characterization of systolic and diastolic function, pulmonary pressures, valvular function, right ventricular function, and the pericardium. Digital storage of images is advisable to allow visual comparison in doubtful cases. The recommended method for 2DE-LVEF quantification is the modified Simpson's biplane method.16 However, due to various factors (reader experience, geometrical assumptions or suboptimal endocardial border definition), 2DE-LVEF has low sensitivity for the detection of small changes in LV function and has a reported test-re-test variability ranging from 9% to 10.8%, which is higher than the threshold used to define CTRCD.22 Contrast agents and automated contour detection may be used to reduce variability. In fact, in the study by Cannesson et al.,23 automated 2DE-LVEF measurements had lower interobserver variability (3.4%) than the manual biplane method (9.8%).
Three-dimensional echocardiography (3DE) provides a compelling alternative with many advantages similar to cardiac magnetic resonance (CMR) imaging.16 It increases the ability to detect smaller changes in LVEF, with a higher reproducibility than 2DE when compared with CMR.24 Three-dimensional echocardiography volume measurements are independent of LV geometric assumptions or apical foreshortening (Table 1). The reduced observer and test-re-test variability in 3DE is at least partially attributable to the automated endocardial tracing.25 In a recent study, Thavendiranathan et al.26 followed up 56 women undergoing chemotherapy by 2DE and 3DE at 3 monthly intervals for 1 year to determine the technique with the least variability. The use of noncontrast 3DE provides lower temporal variability than 2D-LVEF (5.8 vs 9.8%), which is of the utmost importance in these patients.
Imaging Methods for Assessment of Cardiotoxicity
| Advantages | Limitations | |
|---|---|---|
| 2DE-LVEF | Low cost Availability High temporal resolution Wealth of published data | Apex frequently foreshortened Endocardial dropout Blind to shape distortions not visualized in the apical 2- and 4-chamber planes Low detection of subclinical toxicity |
| 3DE-LVEF | No geometrical assumption Unaffected by foreshortening More accurate and reproducible than 2DE-LVEF | Lower temporal resolution Fewer published data on normal values Image quality dependent Low detection of subclinical toxicity |
| GLS | More accurate and reproducible than 2D-LVEF Ability to identify subclinical toxicity Reproducible when performed by trained operators | Availability Vendor- and software-specific Influenced by loading conditions Lack of long-term randomized clinical trials |
| CMR | Most accurate method to measure LVEF Provides assessment of myocardial tissue changes moving the field from depending on functional measures of cardiotoxicity to recognizing the underlying pathological changes | Lack of availability Several contraindications Low temporal resolution Limited data on its use in cardio-oncology |
| Cardiac CT | Noninvasive method to assess CAD High sensitivity and negative predictive value for CAD | Radiation exposure Reduced specificity for obstructive CAD especially in the context of calcified plaque |
2DE, 2-dimensional echocardiography; 3DE, 3-dimensional echocardiography; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CT, computed tomography; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
The reproducibility of 3DE may be of particular importance in patients with low normal LVEF. In 114 adult survivors of childhood malignancies treated with chest radiation and/or anthracyclines, 16 (14%) were found to have LVEF < 50% by CMR as the reference standard, but 10 of the 16 were misclassified by 2DE as having preserved LVEF by the biplane method.27 On average, 2DE overestimated LVEF by 5% (mean LVEF 56% in CMR, 55% in 3DE, and 61% in 2DE by biplane) and had wider ranges and limits of agreement. 3DE-measured LVEF was the most sensitive echocardiography parameter to identify a LVEF < 55% with CMR. Based on these results, it was suggested that 2DE-LVEF at the lower limits of normal (range of 50%-59%) warrants particular attention and may require further cardiac evaluation to rule out cardiac dysfunction.28
The ASE, the EACVI, the European Society of Cardiology and the Canadian Cardiovascular Consensus Statement recommend serial imaging with calculated LVEF by the best method available in an echocardiography laboratory.15,17,18 Today 3DE is the preferred technique for the longitudinal monitoring of LVEF in cancer patients.26,29 Fully automated software decreases 3DE-LVEF measurement variability, is timesaving and will help facilitate the integration of 3DE into clinical practice.30 Operator expertise, standardized approaches, and quality improvement initiatives within the echocardiography laboratory are required to achieve the superiority of 3DE-LVEF.16,24 The latter is particularly important given that changes as small as 10% in LVEF are commonly used to define CTRCD and to initiate cardioprotective therapies.15,17,18
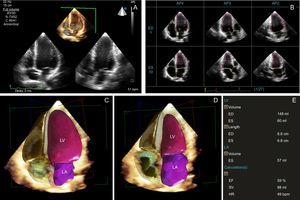
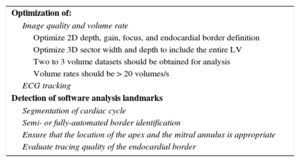
Tips and Tricks for Daily PracticeImage acquisition in 3DE is similar to 2DE with a 1 to 2minute acquisition time from the apical position (Table 2).16,25 Two to 3 volume datasets should be obtained for analysis with the best dataset used for analysis. Volume rates should be greater than 20 volumes per second. Analysis is typically performed offline with semi- or fully automated border identification to delineate the endocardium and epicardium. To integrate 3DE measurements into clinical practice, fully automated software is the logical next step (Figure 1).30
Tips and Tricks for 3D Echocardiography Left Ventricular Ejection Fraction Measurement
| Optimization of: |
| Image quality and volume rate |
| Optimize 2D depth, gain, focus, and endocardial border definition |
| Optimize 3D sector width and depth to include the entire LV |
| Two to 3 volume datasets should be obtained for analysis |
| Volume rates should be > 20 volumes/s |
| ECG tracking |
| Detection of software analysis landmarks |
| Segmentation of cardiac cycle |
| Semi- or fully-automated border identification |
| Ensure that the location of the apex and the mitral annulus is appropriate |
| Evaluate tracing quality of the endocardial border |
2D, 2-dimensional; 3D, 3-dimensional; ECG, electrocardiogram; LV, left ventricle.
Three-dimensional echocardiography (3DE) left ventricular ejection fraction with a fully automated software. A: single-beat full-volume 3DE data sets of the entire left ventricle, performed in the apical 4-chamber view window (B). Users can edit the location of the endocardial border if needed. Final ED (C) and ES frame (D). E: 3D left ventricular volumes and EF. AP2, apical 2-chamber view; AP3, apical 3-chamber view; AP4, apical 4-chamber view; ED, end-diastolic; EF, ejection fraction; ES, end-systolic; HR, heart rate; LA left atrium; LV, left ventricle; SV, stroke volume.
Most efforts to reduce cardiotoxicity are focused on the early diagnosis and treatment of LV dysfunction. However, LVEF is an insensitive measure of early myocardial dysfunction.13 When a patient develops LV dysfunction, particularly with anthracyclines, myocardial damage is established and the chance of recovery, even with evidence-based cardiac therapy, decreases with time.21,31 Whether the same concerns exist with other cancer therapy agents remains to be determined. Emerging data suggest that cardiac biomarkers and new echo techniques may have more sensitivity for the early detection of cardiotoxicity.6,15,17,18
The heart has a helical structure composed of 3 layers of myocardial fibers. Left ventricular systolic function is a coordinated action between them (longitudinal contraction, circumferential shortening, and radial thickening) and ejection fraction predominantly evaluates radial function. Technologies such as speckle-tracking echocardiography (STE) have enhanced the noninvasive assessment of myocardial deformation from conventional 2DE images and provide accurate information in the early phases of myocardial diseases.32 The measurement of deformation is commonly referred to as myocardial strain. Global longitudinal strain (GLS) is the most commonly studied parameter to detect preclinical disease.33 It is highly reproducible when performed by trained operators (inter- and intraobserver variability < 4%),34 but normal ranges are vendor- and software-dependent.35,36 Recognizing the critical need for standardization of strain imaging, the EACVI/ASE invited technical representatives from all interested vendors to participate in a concerted effort to reduce intervendor variability of strain measurement.37 Recently, a study compared head-to-head GLS measurements using 7 different STE software packages, in a group of volunteers with an average LVEF of 60%. Global longitudinal strain values ranged from −18.0% to −21.5%. The inter- and intraobserver reproducibility of GLS proved to be comparable with or superior to that of LVEF (interobserver relative mean errors were 5.4% to 8.6%, while the intraobserver relative mean errors were 4.9% to 7.3%). The absolute difference between vendors for GLS was up to 3.7% strain units (P < .001), lower than for LVEF.38 Although the current recommendation is to use the same vendor for serial surveillance,15,17,18 improvements in the standardization of measures will soon lead to follow-up of patients with different vendor echocardiography machines.39
A growing body of literature supports the use of myocardial strain analysis in patients receiving cancer therapy for baseline evaluation, treatment monitoring, and surveillance of cancer survivors.40
Baseline Cardiotoxicity Risk AssessmentSeveral studies have shown the usefulness of STE-derived strain, as a predictor of outcome in HF patients.33 In cancer patients, GLS has been demonstrated to be superior to LVEF in cardiotoxicity prediction. Prechemotherapy GLS was independently associated with cardiac events at a median follow-up of 4 years. A GLS absolute value < 17.5% was associated with a 6-fold increase in cardiac death or symptomatic HF.41 Prechemotherapy GLS has also been demonstrated to be an effective tool to stratify cardiotoxicity risk in patients with a baseline LVEF between 50% and 59%.42 Recently, circumferential strain was also demonstrated to be strongly predictive of CTRCD.43 These studies open new lines of investigation to noninvasively identify patients at high risk for symptomatic HF before cancer treatment.
Monitoring During Cancer TreatmentIn chemotherapy-treated patients, GLS detects early myocardial dysfunction and predicts CTRCD.40 Although data regarding its ability to predict long-term CTRCD are still lacking, several studies have demonstrated its usefulness in the short-term.44,45 The degree of change in GLS that predicted subsequent cardiotoxicity differed between studies and ranged from 10% to 15%.40 An early study demonstrated that in 45 women with breast cancer treated with trastuzumab/anthracyclines, a relative reduction in GLS of 10% at 3 months predicted subsequent cardiotoxicity (defined as an LVEF < 50% at 6 months).46 In another study of 81 consecutive women prospectively treated with trastuzumab (most also received anthracyclines), 24 (30%) developed cardiotoxicity (defined as a > 10% decline in LVEF from baseline at 12 months). The strongest predictor of cardiotoxicity was a relative decrease in GLS > 11% at 6 months (area under the curve, 0.84; 95% confidence interval, 8.3%-14.6%).47 A combination of biomarkers and GLS increased accuracy for cardiotoxicity diagnosis and if both were negative they identified a group at relatively low risk for CTRCD (negative predictive value of any of these markers for LV dysfunction at follow-up between 91% to 97%).45,47
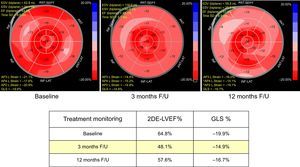
A relative reduction in GLS of > 15% is the threshold defined by ASE to identify subclinical LV dysfunction, whereas a change of < 8% appears not to be of clinical significance. To avoid a false-positive diagnosis of CTRCD, the abnormal value should be confirmed by a repeat study performed 2 to 3 weeks later15 (Figure 2).
Cancer treatment monitoring. A 67-year-old woman with HER2+ breast cancer. The patient had a previous history of smoking and mild hypertension. A tumorectomy was performed and she was subsequently treated with anthracycline-based therapy and trastuzumab. At 3 months’ follow-up, an asymptomatic decrease in GLS and 2DE-LVEF was documented. Under enalapril and carvedilol therapy, cardiac dysfunction improves without treatment interruption, but may not fully recover to baseline. 2DE, 2-dimensional echocardiography; ANT, anterior; AP2 L. Strain, apical 2-chamber view longitudinal strain; AP3 L. Strain, apical 3-chamber view longitudinal strain; AP4 L. Strain, apical 4-chamber view longitudinal strain; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; F/U, follow-up; GLS, global longitudinal strain; INF, inferior; LAT, lateral; LVEF, left ventricular ejection fraction; SD, standard deviation; SEPT, septal.
There is no solid evidence on the most appropriate clinical management when an isolated fall in strain is the only abnormality. Preliminary data support the use of beta-blockers in preventing CTRCD in cancer patients experiencing a significant drop in GLS during treatment.48 However, this will need to be proven prospectively. If a strain-based approach is shown to affect clinical outcomes, this would change the way patients receiving potentially cardiotoxicity cancer therapy are followed up in the future, given that this approach has been shown to be cost-effective.49
Assessment in SurvivorsCardiac follow-up should be undertaken in cancer survivors who have received heart radiation or chemotherapy to enable the detection of cardiovascular toxicities, ideally in the asymptomatic phase of the disease.50 In survivors of childhood cancer, the most common abnormality found in echo follow-up was a significant reduction in the GLS with preserved LVEF (28% of patients with abnormal GLS and LVEF > 50%).5 In the HF population, GLS improves risk stratification especially in patients with LVEF in the low normal or mild depressed ranges.33 Further studies are needed to determine whether this concept applies to cancer survivors and whether a strain-based intervention alters long-term clinical outcomes.
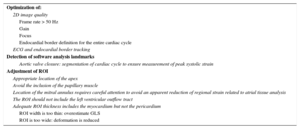
Tips and Tricks for Daily PracticeGlobal longitudinal strain is a sensitive and robust measure to detect subclinical myocardial dysfunction; however, there are no standard guidelines for measuring it adequately, which could contribute to interobserver variability. Experience and training affect the precision and validity of GLS measurement.51 Recently Negishi et al.52 published a set of instructions to help improve reader uniformity in an international multicenter trial of the incremental value of myocardial strain for the detection and management of cardiotoxicity (SUCCOUR ACTRN12614000341628). Table 3 summarizes the steps for STE image acquisition and tips and tricks for daily practice. As the range of normality for different techniques is not interchangeable, the same method should be used to assess GLS during follow-up. Digital storage of images is advisable to allow visual comparison in doubtful cases.
Tips and Tricks for Myocardial Strain Measurement
| Optimization of: |
| 2D image quality |
| Frame rate > 50 Hz |
| Gain |
| Focus |
| Endocardial border definition for the entire cardiac cycle |
| ECG and endocardial border tracking |
| Detection of software analysis landmarks |
| Aortic valve closure: segmentation of cardiac cycle to ensure measurement of peak systolic strain |
| Adjustment of ROI |
| Appropriate location of the apex |
| Avoid the inclusion of the papillary muscle |
| Location of the mitral annulus requires careful attention to avoid an apparent reduction of regional strain related to atrial tissue analysis |
| The ROI should not include the left ventricular outflow tract |
| Adequate ROI thickness includes the myocardium but not the pericardium |
| ROI width is too thin: overestimate GLS |
| ROI is too wide: deformation is reduced |
2D, 2-dimensional; ECG, electrocardiogram; GLS, global longitudinal strain; ROI, region of interest.
Applications of CMR in cardio-oncology include the accurate measurement of LVEF and myocardial tissue characterization.
Cardiac Magnetic Resonance Measured LVEFCardiac magnetic resonance is considered the reference standard for the measurement of ventricular volumes and ejection fraction.53 This is due to its accuracy and superior interobserver, intraobserver, and test-re-test variability.54 Routine use of Cardiac Magnetic Resonance in cardio-oncology is not feasible outside certain selected centers due to the lack of widespread accessibility. However, if available, it would provide a more reliable assessment of small changes in left and right ventricular volumes and function.55,56 Detection of these small changes may represent early markers of myocardial injury. However, this theory has not been prospectively tested. The current practical application of CMR-measured ventricular function in cardio-oncology include: a) measurement of LVEF when there is either a discrepancy between LVEF measurement and clinical symptoms or when there is disagreement between other imaging modalities, and b) identification of subclinical cardiomyopathy in cancer survivors.27
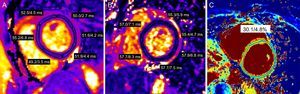
Cardiac Magnetic Resonance Tissue CharacterizationIn addition to accurate LVEF assessment, CMR has the unique ability to detect and quantify pathological myocardial changes noninvasively, making it an invaluable tool in the diagnosis of cardiomyopathies.57–59 Cardiac magnetic resonance techniques for myocardial tissue characterization include early gadolinium enhancement, T2 and T1 weighted imaging or mapping, and late gadolinium enhancement imaging.60 The early gadolinium enhancement technique allows assessment of myocardial inflammation based on the fact that inflammation is associated with myocardial hyperemia and capillary leak.58,61 The increased blood volume in the inflamed areas leads to higher concentration of gadolinium based contrast agents (GBCA) during the early vascular phase. This can be detected by measuring the signal intensity ratio on pre- and early postcontrast T1 weighted fast spin echo images.58,62 Another consequence of myocardial injury is an increase in the permeability of cell membranes followed by a loss of cell membrane integrity.62 This results in intracellular edema initially followed by interstitial edema. These cellular changes can be identified by qualitative T2 weighted or quantitative T2 mapping sequences (Figure 3) with some growing interest in using precontrast T1 mapping sequences as well.57,63–66 An increased T2 tissue signal, or quantitative T2 or T1 values are felt to represent myocardial edema.57,63–70 More recently, a combined use of pre- and postcontrast T1 mapping has been used to calculate the myocardial extracellular volume (ECV) fraction (Figure 3).71 This is based on the fact that with an expansion of the extracellular space due to fibrosis or interstitial edema, the accumulation of extracellular GBCA would be larger, affecting myocardial T1 values.72,73 Considering respective pre-GBCA T1 values, corresponding T1 values of the blood pool and the patient's hematocrit, the ECV fraction can be calculated. Although primarily described to assess diffuse interstitial myocardial fibrosis, myocardial interstitial edema can also increase ECV.72 Finally, late gadolinium enhancement imaging can be used for the identification of replacement fibrosis (scar).74 Gadolinium based contrast agent will accumulate into the expanded extracellular space (due to scar) and reduce the T1 values in this area. This amount of scar can then be identified by T1-weighted inversion recovery.75
Tissue characterization techniques with cardiac magnetic resonance. A and B: short axis T2 maps obtained before ecancer therapy and after anthracycline treatment demonstrating a regional increase in T2 values suggesting myocardial edema. C: extracellular volume map showing marginally elevated extracellular volume in a patient after cancer therapy.
A recent systematic review has outlined the limited literature on the use of CMR tissue characterization in cardio-oncology.76 Existing animal model studies demonstrate that edema detected using T1 and T2 weighted imaging can identify the earliest signs of cardiotoxicity.77,78 A clinical study of 46 women with breast cancer treated with anthracyclines+/-trastuzumab, demonstrated myocardial edema using T2 weighted imaging in 49% of the patients at 1 to 4 months during therapy. Patients with edema were more likely to have persistent reduction in right ventricular function at follow-up.57 Limited data also suggest that with anthracyclines an early increase in early gadolinium enhancement identifies patients who subsequently have a reduction in LVEF. Several studies have also examined myocardial tissue changes in cancer survivors and have demonstrated an increase in myocardial ECV, suggesting diffuse fibrosis. In pediatric cancer survivors the increase in ECV was associated with poor exercise tolerance.79,80 Despite these interesting data, the clinical relevance of these myocardial tissue changes during cancer therapy and in survivors is undefined.
Tips and Tricks for Daily PracticeCardiac Magnetic Resonance is the modality of choice for accurate measurement of LV volumes and function. Its routine application in clinical practice to follow up patients receiving cancer therapy is not practical at present. Its current clinical role is when there is discrepancy in LV function between 2 different modalities, and decisions regarding continuing cancer therapy is contingent on accurate LVEF determine. Myocardial tissue characterization is a potential unique application of CMR particularly for understanding the pathophysiological myocardial changes that underpin the functional changes seen during and after cancer therapy. Several ongoing studies are currently exploring the clinical value of myocardial tissue characterization in patients receiving cancer therapy
CARDIAC COMPUTED TOMOGRAPHYThere are limited data on the use of cardiac computed tomography (CT) in the assessment and management of patients receiving cardiotoxic cancer therapy.81 Although functional assessment can be performed with cardiac CT, it is limited by significantly lower temporal resolution and would not be considered a primary modality for this purpose. Its primary use includes the detection or exclusion of coronary artery disease and pericardial disease. Cardiac CT may have a role in pretreatment risk assessment via the demonstration of coronary calcification to identify subclinical coronary disease in patients with established risk factors. In fact, in the Multiethnic Study of Atherosclerosis (MESA), there was an increased prevalence of coronary atherosclerosis in patients with a new diagnosis of cancer.82 Identification of such risk factors can promote the use of evidence-based therapies, such as statins prior to initiation of cardiotoxic cancer therapy.
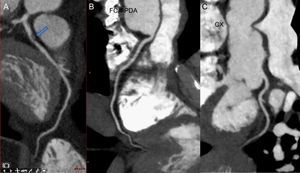
Certain chemotherapeutics, such as antimetabolites, antimicrotuble agents, and tyrosine kinase inhibitors, have been associated with the development of coronary disease and ischemia.83 In addition, radiation therapy to the chest is associated with the development of coronary artery disease.84 Cardiac CT has excellent diagnostic performance in the detection of coronary disease and is well known for its high negative predictive value (Figure 4). In fact, several studies have suggested that the CT coronary angiogram may be an ideal technique for the early detection of radiation-induced coronary artery disease in pediatric cancer survivors.85,86
Certain chemotherapies, as well as radiation therapy, are associated with pericardial disease. Cardiac CT provides exquisite assessment of pericardial effusion, thickening, and pericardial calcification. When pericardial disease is suspected, after physiological assessment using echocardiography or CMR, cardiac CT is an excellent adjunct modality. The use of cardiac CT in the assessment of pericardial disease in general has been previously reviewed.87
Tips and Tricks for Daily PracticeCardiac CT does not have a routine role in cardio-oncology, but its potential use is in the assessment of subclinical or clinical coronary artery disease and in the investigation of potential pericardial disease.
CONCLUSIONSPatients, who undergo cancer treatments, should be considered to be at high risk of cardiovascular complications; the most frequent of which is cardiac dysfunction and HF. Conclusive data regarding the optimal surveillance scheme are lacking and no evidence-based recommendations can be firmly established. Because resources are limited, each center should design its own surveillance algorithm depending on the availability and expertise of each technique. In experienced laboratories, the use of 3DE-LVEF and GLS should be emphasized for serial surveillance during cancer treatment. 3DE is more accurate and reproducible than 2DE for the measurement of LVEF and has the best temporal reproducibility during cancer therapy. The use of GLS to identify subclinical myocardial injury should be implemented particularly in high risk patients. Routine use of CMR to follow up patients receiving cancer therapy is not practical at present. It effective use is when there are discrepancies between different modalities and cancer treatment needs to be withheld. The potential use of cardiac CT is in the assessment of subclinical or clinical coronary artery disease and in the investigation of potential pericardial disease. Depending on the modality and frequency of the surveillance strategy feasible at each center, local cardio-oncology teams should reach a consensus on how to manage patients with suspected CTRCD or subclinical myocardial dysfunction, in order to standardize decisions such as when to start cardioprotective drugs or withhold life-saving cancer therapy.
CONFLICTS OF INTERESTNone declared.