The association between Streptococcus bovis group infective endocarditis and colorectal neoplasm (CRN) is well-known. However, no studies have assessed the association between Enterococcus faecalis infective endocarditis (EFIE) and CRN. We aimed to determine whether the prevalence of CRN is higher in patients with EFIE and an unclear source of infection than in patients with EFIE and a known source of infection or in the general population.
MethodsRetrospective analysis of a cohort of 154 patients with definite EFIE (109 with an unclear source of infection and 45 with an identified source) from 2 Spanish teaching hospitals to determine the prevalence of CRN and other colorectal diseases.
ResultsIn the group with an unknown source of infection, 61 patients (56%) underwent colonoscopy; of these, 31 (50.8%) had CRN. Nonadvanced colorectal adenoma was detected in 22 patients (36%), advanced adenoma in 5 (8.2%), and colorectal carcinoma (CRC) in 4 (6.6%). Among patients who survived the EFIE episode with ≥ 2 years of follow-up, 1 case of CRC was subsequently diagnosed. Only 6 patients (13.3%) with an identified focus of infection underwent colonoscopy; 1 of these patients (16.7%) was diagnosed with CRN. The prevalence of adenomas was slightly higher than that of the Spanish population in the same age range, whereas that of CRC was 17-fold higher.
ConclusionsCRN was found in more than half of patients with EFIE and an unclear focus of infection who underwent colonoscopy. Colonoscopy should be recommended in patients with EFIE and an unclear source of infection.
Keywords
Enterococcus faecalis is the third leading cause of infective endocarditis (IE) worldwide. It is becoming increasingly relevant due to epidemiological changes among patients at risk.1,2E. faecalis is one of the most relevant microorganisms of gut commensal flora involved in human disease. It is located in the colon wall, where it is involved in various processes, including translocation leading to systemic infection.3 However, the most commonly reported portal of entry in the literature on E. faecalis infective endocarditis (EFIE) is the genitourinary tract, followed by unknown origin.4 Some preliminary data indicate that E. faecalis might be involved in the mutagenesis of colonic cells5 and that the presence of lesions affecting the intestinal mucosa may lead to higher rates of translocation of E. faecalis into the bloodstream. However, whereas the relationship between IE caused by Streptococcus bovis group and colon malignancies has been widely studied,6–8 the relationship between EFIE and colorectal neoplasms (CRNs) has not been thoroughly explored to date.
Colonoscopy is the gold standard for CRN screening.9 In a study performed in asymptomatic patients with undiagnosed CRN undergoing colonoscopy for colorectal carcinoma (CRC) screening in Spain, the disease was detected in 27 of 5059 patients undergoing colonoscopy (0.5%) and in 30 patients (4.5%) with positive fecal immunochemical testing and colonoscopy findings (n = 663); advanced colorectal adenomas (CRAs) were found in 493 (9.7%) and 252 (38%) patients and nonadvanced CRA in 1116 (22.1%) and 112 (16.9%) patients, respectively.10 The global prevalence of CRA and CRC differs significantly between men and women (about 30% vs 20% for CRA and 1.4% vs 0.6% for CRC).11,12 More than 70% of EFIE patients are men, with a median age of around 70 years.1,2 The prevalence of CRC among patients within the age range of standard EFIE patients (70-80 years) undergoing screening colonoscopy is 0.8%-3.2%.11
Our aim was to assess the prevalence of CRN, and specifically that of CRC, in patients with EFIE undergoing colonoscopy due to an unclear origin of infection and to compare it with that of patients with an identified portal of entry. We compared this prevalence with that of the Spanish general population to determine whether colorectal examination to rule out neoplasm is necessary in these patients.
METHODSDesignWe performed a descriptive, retrospective analysis of 2 cohorts that were prospectively collected from 2 Spanish teaching hospitals (centers 1 and 2): Hospital Clínic, Barcelona, Spain, an 830-bed university reference center for cardiac surgery (1979 to 2015); and Hospital Lucus Augusti, Lugo, Spain, a 690-bed center (1988 to 2015). The inclusion criteria were definite EFIE according to the modified Duke criteria13 and available clinical and microbiological data. Patients with previous episodes of IE due to S. bovis were excluded due to a higher risk of CRN.
Variables Related to Infective EndocarditisDemographic, microbiological, and clinical data were collected as defined in previous studies by our group.14,15 Antibiotic therapy and surgery were indicated according to American16 and European17 guidelines. Our working group on IE held multidisciplinary weekly meetings to evaluate the management of all suspected or confirmed IE cases during the last 30 years.18
Variables Related to Colorectal NeoplasmsData from colonoscopies performed to investigate the origin of EFIE during admission were collected. Endoscopic findings as reported by gastroenterologists from the 2 centers and histopathological reports of colorectal samples by pathologists were categorized as CRN as follows: nonadvanced CRA was defined as a tubular adenoma with a diameter of < 1cm; advanced CRA was defined as an adenoma with a diameter of ≥ 1cm, or tubulovillous (25%-75% of villous component) or villous (> 75%) histology, or high-grade dysplasia. Carcinoma in situ was classified as an adenoma with high-grade dysplasia. The criterion for invasive cancer was the presence of malignant cells beyond the muscularis mucosa. Colorectal neoplasms included both CRA and CRC. The histological classifications of polyps and CRN were based on World Health Organization criteria.19 Nonneoplastic colonic diseases were also registered.
Performance of Colonoscopy or Other Digestive Tests During Admission for Enterococcus faecalis Infective EndocarditisColonoscopy and other tests to study the gastrointestinal tract have been systematically performed to identify the focus of EFIE in both centers since the late 1990s. Colonoscopy has been the test of choice in Hospital Lucus Augusti since 1998 and in Hospital Clínic only since 2010 (barium enema was previously used). None of the patients undergoing colonoscopy had a high pretest suspicion of CRN. Reasons for not performing colonoscopy were poor clinical status or prognosis irrespective of colonic disease or refusal of the patients or relatives. Thus, not all patients with EFIE and an unclear focus of infection diagnosed during the study period underwent colonoscopy. In some patients, the work-up to determine the focus of the infection could not be completed for various reasons during admission; therefore, we also included patients undergoing colonoscopy and other gastrointestinal tests during the first 3 months after admission.
Statistical AnalysisCategorical variables were compared using the chi-square test (or the Fisher exact test when necessary). Continuous variables were compared using the Kruskal-Wallis test. A 2-sided P < .05 was considered statistically significant. The statistical analysis was performed using SPSS version 16.0 (SPSS Inc.; Chicago, Illinois, United States).
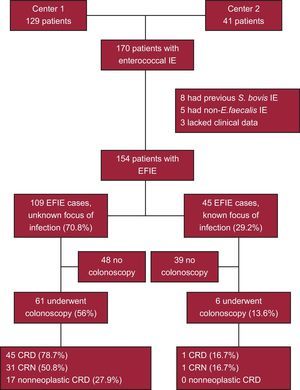
RESULTSWe evaluated 170 patients to determine their eligibility for the study and excluded 16 (Figure): 8 had a previous episode of IE caused by S. bovis and were considered to be at higher risk for CRN at baseline, 5 had non-E. faecalis species enterococcal IE, and 3 lacked clinical data. The study population comprised 154 patients with EFIE: 109 had an unclear source of infection and 45 had an identified portal of entry (genitourinary tract, 34; catheter, 4; surgical wound, 4; biliary tract, 3).
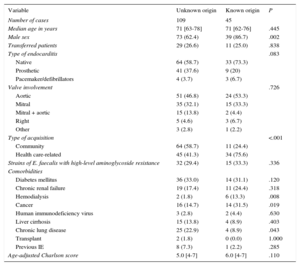
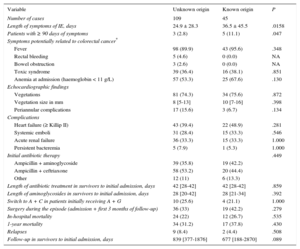
The demographic data of the 154 cases of EFIE included in the analysis are shown in Table 1. The general characteristics correspond to that of a typical EFIE profile: elderly individuals with multiple comorbidities (median age-adjusted Charlson score ≥ 5 in both groups), with a predominance of native and aortic endocarditis. Of note, patients with an unknown origin were more frequently male and had acquired their disease in the community. Patients with a known source had higher rates of cancer and hemodialysis. As for clinical characteristics, treatment details, and outcomes (Table 2), patients with an identified portal of entry had significantly longer symptom duration prior to diagnosis; however, no other significant differences were found between the 2 cohorts.
Baseline Characteristics of 154 Patients With Enterococcus faecalis Infective Endocarditis According to the Source of Infection
| Variable | Unknown origin | Known origin | P |
|---|---|---|---|
| Number of cases | 109 | 45 | |
| Median age in years | 71 [63-78] | 71 [62-76] | .445 |
| Male sex | 73 (62.4) | 39 (86.7) | .002 |
| Transferred patients | 29 (26.6) | 11 (25.0) | .838 |
| Type of endocarditis | .083 | ||
| Native | 64 (58.7) | 33 (73.3) | |
| Prosthetic | 41 (37.6) | 9 (20) | |
| Pacemaker/defibrillators | 4 (3.7) | 3 (6.7) | |
| Valve involvement | .726 | ||
| Aortic | 51 (46.8) | 24 (53.3) | |
| Mitral | 35 (32.1) | 15 (33.3) | |
| Mitral + aortic | 15 (13.8) | 2 (4.4) | |
| Right | 5 (4.6) | 3 (6.7) | |
| Other | 3 (2.8) | 1 (2.2) | |
| Type of acquisition | <.001 | ||
| Community | 64 (58.7) | 11 (24.4) | |
| Health care-related | 45 (41.3) | 34 (75.6) | |
| Strains of E. faecalis with high-level aminoglycoside resistance | 32 (29.4) | 15 (33.3) | .336 |
| Comorbidities | |||
| Diabetes mellitus | 36 (33.0) | 14 (31.1) | .120 |
| Chronic renal failure | 19 (17.4) | 11 (24.4) | .318 |
| Hemodialysis | 2 (1.8) | 6 (13.3) | .008 |
| Cancer | 16 (14.7) | 14 (31.5) | .019 |
| Human immunodeficiency virus | 3 (2.8) | 2 (4.4) | .630 |
| Liver cirrhosis | 15 (13.8) | 4 (8.9) | .403 |
| Chronic lung disease | 25 (22.9) | 4 (8.9) | .043 |
| Transplant | 2 (1.8) | 0 (0.0) | 1.000 |
| Previous IE | 8 (7.3) | 1 (2.2) | .285 |
| Age-adjusted Charlson score | 5.0 [4-7] | 6.0 [4-7] | .110 |
IE, infective endocarditis.
Data are expressed as No. (%) or median [interquartile range].
Clinical and Treatment Characteristics and Outcomes of the 154 Patients With Enterococcus faecalis Infective Endocarditis According to the Source of Infection
| Variable | Unknown origin | Known origin | P |
|---|---|---|---|
| Number of cases | 109 | 45 | |
| Length of symptoms of IE, days | 24.9 ± 28.3 | 36.5 ± 45.5 | .0158 |
| Patients with ≥ 90 days of symptoms | 3 (2.8) | 5 (11.1) | .047 |
| Symptoms potentially related to colorectal cancer* | |||
| Fever | 98 (89.9) | 43 (95.6) | .348 |
| Rectal bleeding | 5 (4.6) | 0 (0.0) | NA |
| Bowel obstruction | 3 (2.6) | 0 (0.0) | NA |
| Toxic syndrome | 39 (36.4) | 16 (38.1) | .851 |
| Anemia at admission (haemoglobin < 11 g/L) | 57 (53.3) | 25 (67.6) | .130 |
| Echocardiographic findings | |||
| Vegetations | 81 (74.3) | 34 (75.6) | .872 |
| Vegetation size in mm | 8 [5-13] | 10 [7-16] | .398 |
| Periannular complications | 17 (15.6) | 3 (6.7) | .134 |
| Complications | |||
| Heart failure (≥ Killip II) | 43 (39.4) | 22 (48.9) | .281 |
| Systemic emboli | 31 (28.4) | 15 (33.3) | .546 |
| Acute renal failure | 36 (33.3) | 15 (33.3) | 1.000 |
| Persistent bacteremia | 5 (7.9) | 1 (5.3) | 1.000 |
| Initial antibiotic therapy | .449 | ||
| Ampicillin + aminoglycoside | 39 (35.8) | 19 (42.2) | |
| Ampicillin + ceftriaxone | 58 (53.2) | 20 (44.4) | |
| Other | 12 (11) | 6 (13.3) | |
| Length of antibiotic treatment in survivors to initial admission, days | 42 [28-42] | 42 [28-42] | .859 |
| Length of aminoglycosides in survivors to initial admission, days | 28 [20-42] | 28 [21-34] | .392 |
| Switch to A + C in patients initially receiving A + G | 10 (25.6) | 4 (21.1) | 1.000 |
| Surgery during the episode (admission + first 3 months of follow-up) | 36 (33) | 19 (42.2) | .279 |
| In-hospital mortality | 24 (22) | 12 (26.7) | .535 |
| 1-year mortality | 34 (31.2) | 17 (37.8) | .430 |
| Relapses | 9 (8.4) | 2 (4.4) | .508 |
| Follow-up in survivors to initial admission, days | 839 [377-1876] | 677 [188-2870] | .089 |
A + C, ampicillin plus ceftriaxone; A + G, ampicillin plus aminoglycoside.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Data on CRN diagnosis in the study cohort are shown in Table 3. Sixty-one patients with an unclear source of infection (56%; 44 men [72.1%] and 17 women [27.9%]; median age, 71 years [interquartile range, 46-68 years]) and 6 patients in those with an identified source underwent colonoscopy during the work-up (13.6%; 5 men [83.3%] and 1 woman [16.7%]; median age, 64.5 years [interquartile range, 63-79 years]). One patient diagnosed with CRC underwent colonoscopy more than 3 months after discharge (at 4 months) due to prolonged recovery in a rehabilitation center. Colorectal diseases were diagnosed in 48 of the 61 patients who underwent colonoscopy (78.7%, 95% confidence interval [95%CI], 68.20%-89.17%) in the group with an unknown source of infection. Colorectal neoplasm was identified in 31 patients who underwent colonoscopy in the group without a clear focus of infection (50.8%, 95%CI, 38.02%-63.62%) and in 1 of the 6 patients (16.7%, 95%CI, 0.00%-4.89%) with a known portal of entry. One male patient with an unknown source of infection and normal colonoscopy findings during the initial admission was diagnosed with CRC 4 months after discharge, giving a total of 5 cases of CRC in 109 patients with EFIE and an unknown source of infection (prevalence of 4.6%) and in 61 patients with colonoscopy results from the first admission (prevalence of 8.2%). The ages of the 5 patients with a diagnosis of CRC were 62, 68, 74, 77, and 81 years. Four of the 5 patients died within 2 years of receiving the CRC diagnosis (median survival time was 94 days), whereas 1 was still alive at the end of 2015 (2730 days of follow-up). Additionally, 12 patients in the unknown source group who did not undergo colonoscopy and 5 in the group with an identified source underwent a barium enema. One patient in the first group was diagnosed with CRA in a colonoscopy subsequent to the admission when the barium enema was carried out, whereas none in the second group were diagnosed with CRN.
Gastrointestinal Diseases Among the 154 Patients With Enterococcus faecalis Infective Endocarditis According to the Source of Infection
| Variable | Unknown origin | Known origin | P |
|---|---|---|---|
| Number of cases | 109 | 45 | |
| Patients with colonoscopy during the admission or within the first 90 days after diagnosis | 61 (56) | 6 (13.6) | <.001 |
| Patients with colonoscopy and diagnosis of colonic disease | 48 (78.7) | 1 (16.7) | .004 |
| Colorectal neoplasms diagnosed using colonoscopya | 31 (28.44) | 1 (2.2) | <.001 |
| Colorectal adenoma | 27 (24.8) | 1 (2.2) | <.001 |
| Nonadvanced adenoma | 22 (20.2) | 0 (0.0) | |
| Advanced adenoma | 5 (4.6) | 1 (2.2) | .193 |
| Colorectal cancer | 4 (3.7) | 0 (0.0) | |
| Nonneoplastic diseases diagnosed using colonoscopyb | 17 (15.6) | 0 (0.0) | .005 |
| New diagnosis of adenocarcinoma during follow-up (after 90 days of admission)c | 1/56 (1.8) | 0/31 (0.0) | .560 |
Data are expressed as no./No. (%).
Overall, 32 cases of CRN were diagnosed in the whole cohort during the time between admission and the 3-month follow-up; the overall prevalence of CRN was 20.8% (95%CI, 14.24%-27.32%), 23.6% in men and 14.6% in women (P = .202). If only CRA was taken into account, the overall prevalence was 19.8% in men and 14.6% in women (P = .436). When only patients with colonoscopy were taken into account, the prevalence of CRN was 51% in men and 38.9% in women (P = .378); in the case of CRA, the prevalence was 42.9% and 38.9% for men and women, respectively (P = .770). In-hospital mortality and 1-year mortality were significantly higher among patients not diagnosed with CRN than among those who were (27.9% vs 6.2%; P = .009; and 38.5% and 12.5%; P = .006, respectively), whereas relapses were not significantly more frequent among patients diagnosed with CRN (8.3% in patients without CRN and 3.1% in patients with CRN; P = .460). When patients with CRN were compared with those who did not have the disease, no significant differences were detected in the classic factors associated with poorer prognosis of IE (type of IE [native/pacemaker vs prosthetic], valve involvement, rates of cardiac surgery, systemic and central nervous system emboli, heart failure, renal failure, persistent bacteremia, periannular complications, and type of antibiotic treatment [ampicillin plus aminoglycoside vs ampicillin vs ceftriaxone]) or in baseline comorbidities (data not shown).
DISCUSSIONThe prevalence of CRN in the group of EFIE patients with an unclear source of infection is substantially higher than that expected for similarly aged patients in the general population, approaching that reported in patients with S. bovis IE. The prevalence of CRN in patients with S. bovis undergoing colonoscopy is approximately 60%,8 and both American16 and European17 guidelines recommend systematic colonoscopy in these patients. Although the prevalence of CRN in the group of EFIE patients with an unknown source of infection was significantly higher than that in those with a clear focus (and this finding is reliable), the data from our study are not conclusive because only 6 of 45 patients with an identified focus underwent colonoscopy.
The prevalence of CRA in a cohort study based on an Austrian national screening colonoscopy program with 44 350 participants was 18.4% (95%CI, 16.7%-20.1%) for women and 32% (95%CI, 30%-34%) for men in the 70–74-year age group,11 whereas it was 44.3% (95%CI, 31.54%-56.98%) in our cohort for patients undergoing colonoscopy (45.5% for men and 41.2% for women) in the group with an unknown source of infection. In the Austrian study, and within the same age group, the prevalence of CRC was 0.7% (95%CI, 0.4%-1.2%) in women and 1.9% (95%CI, 1.4%-2.6%) in men.11 In contrast, it was 8.2% (95%CI, 1.17%-15.22%) in our cohort in patients with available colonoscopy results and 11.4% (95%CI, 1.79%-20.93%) in men. Because all patients with a diagnosis of CRC were men, our findings represent a 1.4-fold higher prevalence of CRA and 6-fold higher rate of CRC than in the Austrian cohort when only male sex is considered. In the case of female patients, the CRA rate was 2.2-fold higher.
Comparison with data on the prevalence of CRN diagnosed by colonoscopy in Spain reveals even greater differences. In the study by Quintero et al.,10 the prevalence of CRC in patients undergoing colonoscopy (with no previous fecal immunochemical testing) was 0.5%, whereas CRA was found in 31.8%. Thus, CRC was almost 17 times more frequent in our cohort (0.5% vs 8.2%) in patients with colonoscopy, whereas CRA was only slightly more frequent (31.8% vs 44.3%). However, the mean age of the participants in the study by Quintero et al. was significantly lower than that of patients in our cohort (59.2 ± 5.5 years vs 69 ± 12.4 years in patients undergoing colonoscopy in our study).
A more recent study showed that the CRC rate among patients undergoing surveillance colonoscopy (follow-up colonoscopy in individuals with abnormal results in the baseline colonoscopy) was 9.5 cases per 10 000 persons-years in patients aged 70 to 74 years.20 Thus, even if we considered only the CRC cases diagnosed during follow-up, the incidence of CRC would still be considerably higher in our cohort of EFIE patients with an unknown source of infection undergoing colonoscopy than in patients at higher baseline risk of CRC due to a previous abnormal colonoscopy result within the same age range.
The diagnosis of CRN was not associated with poorer outcomes during admission and at 1 year or with a higher relapse rate. In contrast, mortality was significantly higher in the group without CRN, likely because a relevant percentage of patients presenting with severe courses of IE or poor baseline status did not undergo colonoscopy and thus were not diagnosed with CRN. The absence of an impact of EFIE on the prognosis of CRN is consistent with data from patients with IE due to the S. bovis group, who rarely die of CRN-related complications.15 On the other hand, EFIE and the S. bovis group IE might constitute, respectively, late and early markers of CRC due to the different temporal patterns of presentation. However, the differences between both entities could be due to a wide variety of reasons (eg, the epidemiology of our centers; the recent switch of EFIE from a subacute, community-acquired entity to an acute, health care-associated infection; and nonsystematic performance of colonoscopy in EFIE until recent years). These findings require confirmation in further studies. In this regard, we found a shorter duration of symptoms in patients with EFIE with unknown origin than in those with an identified focus of infection; community acquisition was more frequent in the former, and health care-associated acquisition was more frequent in the latter. We hypothesize that the diagnosis of IE was reached later in patients with health care-associated acquisition and an identified focus because they were mainly patients with invasive urinary procedures or a chronic urinary catheter with recurrent urinary infections. These cases of EFIE were partially treated as urinary infections, thus hindering the early detection of E. faecalis in blood cultures and suspicion of IE.
In our cohort, 17 patients with EFIE and an unknown source of infection underwent barium enema, and none were diagnosed with colorectal diseases. Given the high prevalence of colorectal diseases found in these patients using colonoscopy, we conclude that the sensitivity of barium enema is very low. Even though barium enema is no longer used as a screening tool for CRN in most countries, it remains crucial to highlight that the test of choice for ruling out CRN in patients with EFIE should always be colonoscopy.
Our findings could have a considerable impact not only on the diagnostic assessment of EFIE, but also on its management. If possible, colonoscopy should be performed early after the diagnosis of EFIE because the presence of an advanced neoplasm might change the performance or timing of invasive procedures (namely, cardiac surgery, which could be cancelled despite being indicated or postponed until after the initial treatment of the CRC). However, due to the risk of fluid overload in frail patients with valve regurgitation, as well as the difficulties of performing colonoscopy in critically ill patients, it is unlikely that all patients with EFIE will undergo colonoscopy in an early stage of the episode. In 3 of the patients of our cohort undergoing 18F-FDG positron emission tomography/computed tomography (PET/CT) (performed in most patients with possible or definite IE in Hospital Clínic since 2013) in the initial phase of the diagnostic process, focal colonic uptake aroused early suspicion of CRN, which was later confirmed by colonoscopy and histopathological findings (Supplementary material). 18F-FDG PET/CT is efficacious in IE, not only for diagnosis of prosthetic valve endocarditis, but also for early detection of septic embolisms and neoplasms.21,22 However, 18F-FDG PET/CT cannot replace colonoscopy as a diagnostic tool for CRN in these patients but can only act as an additional approach for confirmation and detection of metastases from the neoplasm. This is especially relevant when treating patients with a high risk of CRN and comorbidities, such as patients with EFIE, because it might determine operability and timing in patients with an indication for cardiac surgery. These “special situations” in IE have not been addressed by recent American16 and European17 guidelines or in the comments of the Spanish Society of Cardiology on the European guidelines.23 As stated elsewhere, we advocate including these considerations in future guidelines and recommendations.24
LimitationsThe present study has several limitations. First, because it is retrospective and colonoscopy was not systematically indicated throughout the study period, endoscopic findings are not available for all patients. Second, the frequency of colonoscopy in our inner control group (patients with an identified focus of infection other than the digestive tract) was very small; therefore, findings in this group could be due to chance and are not conclusive. Third, the prevalence of CRN in our study is compared with that of a historical series of Spanish individuals undergoing colonoscopy for CRC screening. Fourth, the low rate of events related to CRC and mortality precludes any generalization of our results until new studies provide larger and better-controlled data. Fifth, the study period was very long. Consequently, because the rate of EFIE varied throughout and the exact rates of EFIE are unknown in our setting, we were unable to calculate the incidence density of CRN and adjust risk for age groups. Sixth, the availability and development of the different techniques and the expertise necessary to perform them (eg, diagnosis of IE or colonoscopy) have changed over time. Finally, because autopsy was performed in very few cases (CRC was not found in any of them), we cannot provide data on the cause of death (IE, CRN, or other) in patients without colonoscopy results.
CONCLUSIONSIn conclusion, the prevalence of CRN in EFIE with unknown origin was 50.8% in patients undergoing colonoscopy. When compared with patients with EFIE and an identified focus of infection, the CRN rate was significantly higher in the group of patients with an unclear source of infection. The prevalence of CRN was also higher than in the general population. Colorectal adenoma was only slightly higher than in the Spanish population, whereas CRC was 17-fold higher. In light of these preliminary data, colonoscopy should be recommended in all patients with EFIE and an unclear source of infection until further studies on the relationship between EFIE and CRC are available.
FUNDINGThis work was supported in part by a grant from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Madrid, Spain), the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), and the “Fundación Máximo Soriano Jiménez” (Barcelona, Spain). While the manuscript was being drafted, J.M. Pericàs received an “Emili Letang” Postresidency Scholarship (2013-14) from Hospital Clínic, Barcelona (Spain) and a “Río Hortega” Research Grant (CM14/00135; 2015-16) from Instituto de Salud Carlos III and the Ministerio de Economia and Competitividad, Madrid (Spain). J.M.M. received a personal intensification research grant #INT15/00168 during 2016 from Instituto de Salud Carlos III, Madrid, Spain. The European Regional Development Fund (ERDF) “A way to build Europe” also provided funding”. None of the sponsors/funders had a significant role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
CONFLICTS OF INTERESTJ.M. Miró has received consulting honoraria and/or research grants from AbbVie, Bristol-Myers Squibb, Cubist, Novartis, Gilead Sciences, and ViiV. F. Marco has received consulting honoraria from Novartis and Janssen-Cilag. C.A. Mestres has received consulting honoraria from Novartis and Edwards Lifesciences LLC.
- –
Although the relationship between Streptococcus bovis group IE and colorectal cancer is well known, the interplay between EFIE and CRNs has not been thoroughly assessed to date.
- –
The epidemiology of EFIE has changed dramatically during the last 20 years and is now mainly health care-acquired when it is linked to a clear focus.
- –
E. faecalis IE mostly occurs in elderly patients with no identified origin. In these cases, the infection likely has a colorectal origin, although it is seldom identified.
- –
In this clinical setting, colonoscopy is an essential tool for work-up and colorectal cancer screening.
- –
Although preliminary, our findings draw attention to several aspects of the relationship between EFIE and colorectal diseases that have not been previously assessed.
- –
First, there was a predominance of patients with an unclear focus of infection. Second, CRNs were found in more than half of all patients undergoing colonoscopy. Third, the prevalence of CRC was 17 times higher than in the general population of the same age.
- –
This is the first study to provide evidence that colonoscopy should be systematically performed in patients with EFIE and an unclear focus of infection.
Members of the Hospital Clínic Endocarditis Study Group Barcelona, Spain: José M. Miró, Juan M. Pericàs, Asunción Moreno, Carlos Cervera, Adrián Téllez, Juan Ambrosioni, Ximena Castañeda, José M. Gatell (Infectious Diseases Service), Cristina García de la Mària, Yolanda Armero, Javier García-González (Experimental Endocarditis Laboratory), Francesc Marco, Manel Almela (Microbiology Service), Carlos Falces, José M. Tolosana, Bàrbara Vidal, J. Carlos Paré, Manel Azqueta, Marta Sitges (Cardiology), Eduard Quintana, Elena Sandoval, Carlos A. Mestres, Ramón Cartañá, Salvador Ninot, Daniel Pereda, M. Castellà, José L. Pomar (Cardiovascular Surgery), José Ramírez, Teresa Ribalta (Department of Pathology), Mercè Brunet (Toxicology Service), Dolors Soy (Pharmacy Service), David Fuster, Ulises Granados (Nuclear Medicine Service), Jaume Llopis (Statistician from the Departamento de Estadística, Facultat de Biologia, Universitat de Barcelona), and Antoni Castells (Gastroenterology Service).
Investigators from Hospital Lucus Augusti, Lugo, Spain: M. José García País, Ramón Rabuñal, M. José Perez Álvarez, Juan Corredoira (Infectious Diseases Service), Julia Pita, Amparo Coira, Ana Rodríguez Macias, Fernando García Garrote, M. Pilar Alonso (Microbiology Service), and Eva Martí, David Dacal, Beatriz Álvarez (Gastroenterology Service).
The data presented in this study were reported in part at the XIX Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) in Seville, Spain, May 28-30, 2015 and at the 13th International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections of the International Society of Cardiovascular Infectious Diseases (ISCVID), Rio de Janeiro, Brazil, June 4-6, 2015.
See Appendix for a complete list of the Hospital Clínic and Hospital Lucus Augusti Infective Endocarditis Investigators.