The most effective antithrombotic therapy for preventing ischemic complications while limiting bleeding risk in patients with an acute coronary syndrome (ACS) who are undergoing a percutaneous coronary intervention (PCI) is strongly debated.1,2
Bivalirudin and unfractionated heparin (UFH) are 2 of the most commonly used treatment regimens and have been compared in different trials.1 Unfractionated heparin used to be the only anticoagulant drug used before and during PCI in ACS patients. The main advantage of using UFH is that it can be antagonized with intravenous protamine sulfate. However, UFH has some limitations including: a) the need for activated clotting time monitoring due to its variable dose-response relationship with poor predictable effects, b) platelet activation, and c) risk of heparin-induced thrombocytopenia (HIT) and HIT-thrombosis syndrome. Consequently, in the last few years, new anticoagulants with more pharmacologic and clinical advantages than UFH have been tested, although UFH still remains the standard of care for thrombosis prevention during PCI.1
Bivalirudin is a 20 amino-acid synthetic polypeptide that binds directly to thrombin thus inhibiting its enzymatic activity. Bivalirudin cannot be antagonized, but due to its short half-life, its effects are limited by stopping its infusion, and activated clotting time monitoring is not mandatory to verify the effects. Furthermore, bivalirudin does not activate platelets and does not cause HIT given that it does not interact with plasma proteins or cells.1 In recent years, bivalirudin has emerged as an intriguing alternative to UFH, with the main advantage being the lower rates of major bleeding in patients with ACS, particularly in those with ST-segment elevation myocardial infarction (STEMI).3–6 However, some concerns about bivalirudin use have been raised in terms of stent thrombosis (ST).3,5,7,8
Recently, interest in comparing these 2 drugs has been further increased due to the publication of the MATRIX trial.9 This is the latest and largest study comparing bivalirudin and UFH in the setting of ACS. In a contemporary clinical practice (high percentage of revascularization, patients equally balanced to radial and femoral approach, UFH arm without routine use of glycoprotein IIb/IIIa inhibitors (GPI), prehospital antithrombotic treatment, inclusion of new platelet P2Y12 antagonists), this trial showed that the rates of major adverse cardiovascular events and net adverse clinical events were not significantly lower in ACS patients treated with bivalirudin compared with those treated with UFH at 30-days. Notably, bivalirudin was associated with lower rates of major bleeding and mortality but higher rates of ST as previously shown in some of the other trials, although these observations are limited by statistical considerations since the trial was not powered to explore these single endpoints. However, given the previous results of HORIZONS-AMI3 and BRIGHT4 trials, it will be interesting to see if the results of MATRIX will be confirmed or not during a longer follow-up.
In the article published in Revista Española de Cardiología, Verdoia et al10 report the results of a systematic review and meta-analysis of randomized clinical trials comparing bivalirudin and UFH in patients with ACS. The study included 12 randomized clinical trials and 32 746 patients. The 12 trials were categorized as subgroups for clinical presentation (5 for STEMI and 6 for non—ST-segment elevation acute coronary syndrome [NSTEACS] and 1, MATRIX, divided the subgroups into both). Outcomes were analyzed at 30 days, although 1 trial provided in-hospital (SWITCH III) data and 1 at 48hours after discharge (PROTECT-TIMI30). At 30 days, there were no significant differences in all-cause mortality (odds ratio [OR]=0.91; 95% confidence interval [95%CI], 0.77-1.08; P = .28) without significant overall heterogeneity (I2 = 4%). This result was consistent between subgroups of STEMI and NSTEACS (interaction P = .12), although a significant heterogeneity was observed (I2 = 58%) with an opposite trend: NSTEACS OR 1.13 (95% CI, 0.82-1.55); STEMI OR 0.84 (95% CI 0.69-1.02). Stent thrombosis was higher in the bivalirudin treated patients (OR=1.42; 95% CI 1.09-1.83; P = .008), although this result was calculated from only 8 of 12 trials and it is not clear if it referred to definite or to definite/probable ST. Interestingly, some heterogeneity emerged between subgroups (I2 = 45%) with ST being significantly increased only in the 5 STEMI trials analyzed (MATRIX data are missing) but not in the 3 NSTEACS trials. Bivalirudin was found to be associated with lower rates of major bleeding (OR=0.60; 95% CI, 0.54-0.67; P < .0001), consistently in STEMI and NSTEACS trials. However, a high heterogeneity emerged in the latter analysis (I2 = 71%) and the meta-regression analysis demonstrated that this finding was significantly driven by the differential use of GPI with bivalirudin benefits observed in trials with higher use of GPI (P = .02). The study did not explore whether the use of GPI might have influenced mortality or ST. Similarly, there was no exploration of other outcomes (ie, cardiac mortality, myocardial infarction or composite endpoints) as well as the impact of other relevant differential factors (ie, access site or use on new P2Y12 inhibitors).
Systematic reviews and meta-analyses identify, appraise and synthesize all evidence on a specific research question. They allow an increase in the statistical power of treatment comparisons beyond that of individual studies. Thus, meta-analyses are considered the highest level of evidence and could contribute to help physicians stay up to date and to guide healthcare decisions in daily clinical practice.
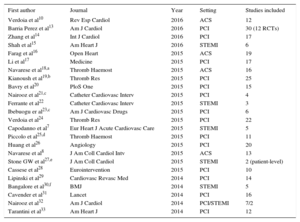
However, the increasing popularity of these studies has led to duplicate meta-analyses on the same topic, sometimes with different results, which make their interpretation difficult for readers.11 Notably, a recent study showed that more than half of meta-analyses have at least 1 overlapping meta-analysis, and some topics had up to 13 overlapping meta-analyses.12 This is exactly what has happened in the comparison between bivalirudin and UFH. When making a search in PubMed, in which we restricted the search to publications from 2014 onward, we found that 24 meta-analyses have been published on this topic (Table).7,8,10,13–33 Although there were some differences among these 24 studies, this is probably one of the more appropriate examples of overlapping meta-analysis on the same clinical question, which significantly contributes to confusion among readers.
Meta-analyses on Bivalirudin vs UFH Appearing in PubMed From 2014 to 1st April 2016
| First author | Journal | Year | Setting | Studies included |
|---|---|---|---|---|
| Verdoia et al10 | Rev Esp Cardiol | 2016 | ACS | 12 |
| Barria Perez et al13 | Am J Cardiol | 2016 | PCI | 30 (12 RCTs) |
| Zhang et al14 | Int J Cardiol | 2016 | PCI | 17 |
| Shah et al15 | Am Heart J | 2016 | STEMI | 6 |
| Farag et al16 | Open Heart | 2015 | ACS | 19 |
| Li et al17 | Medicine | 2015 | PCI | 17 |
| Navarese et al18,a | Thromb Haemost | 2015 | ACS | 16 |
| Kianoush et al19,b | Thromb Res | 2015 | PCI | 25 |
| Bavry et al20 | PloS One | 2015 | PCI | 15 |
| Nairooz et al21,c | Catheter Cardiovasc Interv | 2015 | PCI | 4 |
| Ferrante et al22 | Catheter Cardiovasc Interv | 2015 | STEMI | 3 |
| Ibebuogu er al23,c | Am J Cardiovasc Drugs | 2015 | PCI | 6 |
| Verdoia et al24 | Thromb Res | 2015 | PCI | 22 |
| Capodanno et al7 | Eur Heart J Acute Cardiovasc Care | 2015 | STEMI | 5 |
| Piccolo et al25,d | Thromb Haemost | 2015 | PCI | 11 |
| Huang et al26 | Angiology | 2015 | PCI | 20 |
| Navarese et al8 | J Am Coll Cardiol Intv | 2015 | ACS | 13 |
| Stone GW et al27,e | J Am Coll Cardiol | 2015 | STEMI | 2 (patient-level) |
| Cassese et al28 | Eurointervention | 2015 | PCI | 10 |
| Lipinski et al29 | Cardiovasc Revasc Med | 2014 | PCI | 14 |
| Bangalore et al30,f | BMJ | 2014 | STEMI | 5 |
| Cavender et al31 | Lancet | 2014 | PCI | 16 |
| Nairooz et al32 | Am J Cardiol | 2014 | PCI/STEMI | 7/2 |
| Tarantini et al33 | Am Heart J | 2014 | PCI | 12 |
ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; RCTs, randomized clinical trials; STEMI; ST-segment elevation myocardial infarction; UFH, unfractionated heparin.
A further relevant consideration on the performance of a meta-analysis concerns its methodology. The main principle to pool different studies in a single analysis is that the data/studies included should be similar (study design, eligibility criteria, type of patients and procedures, type of outcomes and their definitions, etc). Are we seeing an analysis of apples and oranges in the comparison of bivalirudin and UFH in ACS or PCI?34 When considering the studies included, one could argue that clinical characteristics and clinical practice differ strongly among them. Trials including different clinical presentations (STEMI, NSTEMI, unstable angina) are pooled together and this is per se a relevant confounding factor. Nevertheless, the TENACITY trial was included in the meta-analysis, even though a quarter of patients underwent elective PCI. If we focus on the 5 trials on STEMI patients and the MATRIX subgroup of STEMI patients, important differences should be considered when pooling these studies together and interpreting the overall results: a) the bivalirudin regimen of post-PCI infusion significantly varied across trials, from being not routinely adopted in the HORIZONS-AMI, BRAVE-4 (Bavarian Reperfusion Alternatives Evaluation-4)35 and HEAT-PPCI,36 to being used in most of the patients in the EUROMAX trial5 (low-dose or high-dose left at the operator's discretion, being finally used in 77.5% and 22.5%, respectively) and BRIGHT (only high-dose allowed) or randomized in the MATRIX to post-PCI infusion or no infusion (low-dose or high-dose left at the operator's discretion, being used in approximately 63% and 37% respectively); b) GPI use differed markedly across trials (HORIZONS-AMI: 7.5% and 97.7% in the bivalirudin and UFH groups; EUROMAX: 11.5% and 69.1%; BRIGHT: 4.4% and 52.9%; HEAT-PPCI: 13.5% and 15.5%; BRAVE-4: 3.0% and 6.1%; MATRIX: 4.6% and 25.9%); c) access site for PCI (almost all femoral access in HORIZONS-AMI and BRAVE-4; three-quarters radial access in BRIGHT and HEAT-PPCI; well balanced in EUROMAX and MATRIX); d) new potent P2Y12 inhibitors (prasugrel or ticagrelor) were frequently used in EUROMAX, HEAT-PPCI, BRAVE-4 (all patients received prasugrel in the bivalirudin arm while all UFH patients received clopidogrel) and MATRIX, but not in HORIZONS-AMI or BRIGHT; e) the UFH dose adopted in heparin monotherapy-treated patients ranged from 70 U/kg in HEAT-PPCI to 100 U/kg in EUROMAX and BRIGHT with the first trial not showing that bivalirudin reduced major bleeding compared with the other trials; f) the HEAT-PPCI, which was the only trial to support UFH advantages over bivalirudin, enrolled all-comer patients but was the only single-center trial; and g) EUROMAX also included 8.5% of patients treated with enoxaparin in the control group. Similarly, substantial differences also can be found in the 6 trials classified as NSTEACS, such as clinical practice and definitions (for example the BAS [Bivalirudin Angioplasty Study] trial was published in 199537 and was reanalyzed in 200138 and enrolled patients in 1993-1994).
In addition to the obvious limitations related to the different designs and patient characteristics of the primary trials included, it should also be considered that pooled data from publications do not offer the opportunity to adjust, and—most of all—published data are sometimes managed or pooled differently from the original design of the trial. In the present analysis, for example, the BRIGHT trial, which randomized patients to 3 arms, was considered as 2-arm by pooling the UFH and UFH+GPI arms. Moreover, the MATRIX trial enrolled all ACS patients and for the present analysis its results were divided for STEMI and NSTEACS. Although sensitivity and meta-regression analyses often help to explore sources of heterogeneity in standard meta-analyses, only patient-level data overcome common limitations by improving internal validity and allowing time-to-event, subgroup, and covariable adjusted analyses.
The results of the present meta-analysis by Verdoia et al seem to be in line with previous literature regarding the benefits of bivalirudin on major bleeding and its risks in terms of ST. However, it is also important to stress that the main increase in ST related to bivalirudin use has been commonly described in acute rather than subacute ST5–8 and that the post-PCI infusion of bivalirudin appears to reduce ST risks.4,5 Future detailed subanalyses from the MATRIX trial could help to clarify this issue because this trial randomized patients to receive the bivalirudin post-PCI infusion or not and also included a large number of patients treated with the 2 different infusion regimens.9 Verdoia et al, however, found no significant differences between bivalirudin and UFH in terms of mortality. The major doubt that remains unresolved is exactly the impact of the drug on mortality: is bivalirudin able to reduce all-cause and cardiovascular death? Major bleeding is an important prognostic determinant of mortality, but all-cause death seems nonsignificantly decreased despite the reduction of major bleeding events.8,10 Interestingly, bivalirudin seems to offer benefits in mortality only in patients with STEMI undergoing primary-PCI, as shown in this and other meta-analyses.7,10,15 However, this apparent differential impact on STEMI and NSTEACS could also be related to the characteristics and differences among the trials included in the analysis. Indeed, the most contemporary MATRIX trial demonstrated consistent results between ACS subgroups.9 Overall, the absence of mortality benefits here described reinforces the concept that reducing bleeding, even the most severe bleeding events, does not necessarily translate into a reduction of major adverse cardiovascular events. It is possible that the increase of ST, as well as a trend toward an increase of myocardial infarction and target vessel revascularization, could mitigate the advantages in bleeding events, which would help to explain the absence of differences in composite events and the uncertainties on mortality benefits.7,9,15
Finally, the cost-effectiveness of choosing bivalirudin rather than UFH should be considered. Are the overall results sufficient to justify the use of bivalirudin despite its much more higher cost?
Some important suggestions for readers have been previously published to aid understanding and to contrast uncertainties when overlapping meta-analyses obtain discordant conclusions.11
In conclusion, do we finally have a winner between bivalirudin and UFH? Although well conducted, this meta-analysis does not provide a definitive conclusion and practitioners still need to use clinical judgment in deciding between a costly bleeding-saving treatment strategy and the costless standard of care, consisting of UFH and limited use of GPI. It remains to be understood whether the use of bivalirudin translates into a real mortality advantage and whether prolongation of infusion after PCI in a full PCI regimen mitigates the risks of ST without trade-offs. While research is still on-going to tease out these outstanding questions, the market penetration of bivalirudin will be probably more affected by the affordability of generic bivalirudin formulations than by its scientific merits.
CONFLICTS OF INTERESTNone declared.