Currently, more than half of patients with congenital heart defects undergo correction procedures in the first year of life and consequently mortality rates are now lower for these patients than in past decades.1 Some patients, however, still require several interventions at older ages due to the use of certain materials for the repair procedures. New products are under assessment with the aim of reducing the number of interventions. One of the most extensively used materials is extracellular matrix, and CorMatrix (CorMatrix Cardiovascular, Inc.; Alpharetta, Georgia, United States) is one of the matrices with the most extensive clinical and experimental experience.2,3
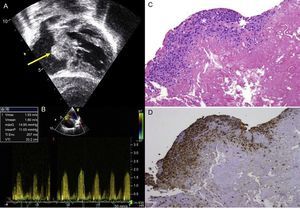
Between October 2010 and June 2014, we used this matrix in our group in 30 patients (Table). These patients were followed-up with serial echocardiography. The mean age of the patients at the time of surgery was 6 months (range, 1 month to 3.8 years) and the mean weight was 7.5kg (range, 3.5-14.5kg). No patients were lost to follow-up, which had a mean duration of 268 days (range, 194.5-305.8 days). No patients required repeat interventions during their hospital stay. No patients died in the postoperative period. Two patients, in contrast, required an intervention during follow-up. One of these was patient 7, who had been diagnosed with situs inversus, single ventricle, pulmonary atresia, and infradiaphragmatic total anomalous pulmonary venous return. He underwent an intervention as a newborn with correction of the anomalous venous return and a central fistula. At 9 months, he underwent a bidirectional Glenn procedure and pulmonary artery plasty with a CorMatrix patch. One month after discharge, he was admitted once more because of labored breathing and desaturation caused by right pleural effusion. Echocardiography showed stenosis at the point where the Glenn shunt joined the corrected pulmonary artery. This was treated with angioplasty, with a good outcome. The second case was patient 9, who had complete atrioventricular canal defect, corrected at the age of 5 months by a double-patch technique. Four months after the intervention, the patient attended the cardiologist, who observed substantial hepatomegaly. The echocardiography showed a mass in the right atrium adhered to the atrial septum that caused severe tricuspid stenosis (Figure A and B). In the following intervention, a neoformative tissue was found in the area of the atrial septum. This tissue occupied a large part of the right atrium. The pathology examination showed vascularized connective tissue with inflammatory foci consistent with foreign-body reaction and an extensive component of fibrin deposits or leukocytes and histiocytes on the surface (Figure C and D), with no evidence of calcification or other cell populations indicative of tissue remodeling.
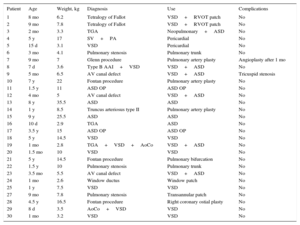
Demographic and Diagnostic Data and Uses and Complications of CorMatrix
| Patient | Age | Weight, kg | Diagnosis | Use | Complications |
|---|---|---|---|---|---|
| 1 | 8 mo | 6.2 | Tetralogy of Fallot | VSD+RVOT patch | No |
| 2 | 9 mo | 7.8 | Tetralogy of Fallot | VSD+RVOT patch | No |
| 3 | 2 mo | 3.3 | TGA | Neopulmonary+ASD | No |
| 4 | 5 y | 17 | SV+PA | Pericardial | No |
| 5 | 15 d | 3.1 | VSD | Pericardial | No |
| 6 | 3 mo | 4.1 | Pulmonary stenosis | Pulmonary trunk | No |
| 7 | 9 mo | 7 | Glenn procedure | Pulmonary artery plasty | Angioplasty after 1 mo |
| 8 | 7 d | 3.6 | Type B AAI+VSD | VSD+ASD | No |
| 9 | 5 mo | 6.5 | AV canal defect | VSD+ASD | Tricuspid stenosis |
| 10 | 7 y | 22 | Fontan procedure | Pulmonary artery plasty | No |
| 11 | 1.5 y | 11 | ASD OP | ASD OP | No |
| 12 | 4 mo | 5 | AV canal defect | VSD+ASD | No |
| 13 | 8 y | 35.5 | ASD | ASD | No |
| 14 | 1 y | 8.5 | Truncus arteriosus type II | Pulmonary artery plasty | No |
| 15 | 9 y | 25.5 | ASD | ASD | No |
| 16 | 10 d | 2.9 | TGA | ASD | No |
| 17 | 3.5 y | 15 | ASD OP | ASD OP | No |
| 18 | 5 y | 14.5 | VSD | VSD | No |
| 19 | 1 mo | 2.8 | TGA+VSD+AoCo | VSD+ASD | No |
| 20 | 1.5 mo | 10 | VSD | VSD | No |
| 21 | 5 y | 14.5 | Fontan procedure | Pulmonary bifurcation | No |
| 22 | 1.5 y | 10 | Pulmonary stenosis | Pulmonary trunk | No |
| 23 | 3.5 mo | 5.5 | AV canal defect | VSD+ASD | No |
| 24 | 1 mo | 2.6 | Window ductus | Window patch | No |
| 25 | 1 y | 7.5 | VSD | VSD | No |
| 27 | 9 mo | 7.8 | Pulmonary stenosis | Transannular patch | No |
| 28 | 4.5 y | 16.5 | Fontan procedure | Right coronary ostial plasty | No |
| 29 | 8 d | 3.5 | AoCo+VSD | VSD | No |
| 30 | 1 mo | 3.2 | VSD | VSD | No |
AAI, aortic arch interruption; AoCo, aortic coarctation; ASD, atrial septal defect; AV, atrioventricular; OP, ostium primum; PA, pulmonary atresia; RVOT, right ventricular outflow tract; SV, single ventricle; TGA, transposition of the great arteries; VSD, ventricular septal defect.
A: Transthoracic echocardiography showing a mass in the right atrium adhered to the atrial septum (arrow) in a patient who underwent correction of an atrioventricular canal defect. B: Transthoracic echocardiography, transtricuspid gradient. C: Pathology study (hematoxylin-eosin) in which predominantly histiocyte inflammatory infiltrate is observed (asterisk). D: Pathology study (CD68 macrophage marker). maxG, maximum gradient; meanP, mean gradient; Ti Env, envelope time; Vmax, maximum velocity; Vmean, mean velocity; VTI, velocity-time integral.
In our experience, CorMatrix appears to be a manageable material with good hemostatic properties. However, our group has faced 2 important complications associated with its use. The first complication was stenosis after pulmonary artery plasty following bidirectional Glenn surgery. The lack of pulsed flow in a pulmonary artery with laminar flow after a Glenn procedure may contribute to the greater inflammatory response of the matrix, thereby obstructing the vessel lumen. Alternatively, the inflammatory reaction itself may reduce the internal diameter after extension of a pulmonary artery such that reduction in flow impedes optimal growth. In the second patient, the onset of tricuspid stenosis secondary to thickening of the septum was caused by an excessive inflammatory response secondary to the foreign body reaction with no evidence of tissue regeneration associated with CorMatrix.
All reports of this product to date in pediatric patients mention complications associated with its use. We note the study by Zaidi et al,4 who published a nonrandomized comparative study of histological assessment after explantation of CorMatrix used in valve repair. In the patients in that study, the use of CorMatrix was associated with an intense inflammatory response that included eosinophils and giant cells, with no evidence of remodeling. The authors postulated that the differences found compared with experimental studies could be the result of implanting the material in congenitally abnormal tissue, which might have lacked certain molecules that would have favored tissue growth. These authors also noted that another mechanism may have been a different inflammatory response which caused the matrix in humans triggered by anti-GAD (decarboxylase glutamate) antibodies.
Our group was unable to demonstrate benefit in terms of the regenerative capacity compared with other patches. However, an inflammatory response has been identified that is consistent with foreign body reaction.
Although our series included a small number of cases and follow-up was less than 1 year, the presence of substantial comorbidity along with other evidence suggests that this material should not be used in children.