Emergency care systems have been created to improve treatment and revascularization in myocardial infarction but they may also improve the management of all patients with acute coronary syndrome.
MethodsA comparative study of all patients admitted with acute coronary syndrome before and after implementation of an infarction protocol.
ResultsThe study included 1210 patients. While the mean age was the same in both periods, the patient group admitted after implementation of the protocol had a lower prevalence of diabetes mellitus and hypertension but more active smokers and higher GRACE scores. The percentage of ST-segment elevation acute coronary syndrome (29.8%-39.5%) and coronary revascularizations (82.1%-90.1%) significantly increased among patients admitted with acute coronary syndrome, and primary angioplasty became routine (51.9%-94.9%); there was also a reduction in time to catheterization and an increase in early revascularization. The mean hospital stay was significantly shorter after implementation of the infarction protocol. In-hospital mortality was unchanged, except in high-risk patients (38.8%-22.4%). After discharge, no differences were observed between the 2 periods in cardiovascular mortality, all-cause mortality, reinfarction, or major cardiovascular complications.
ConclusionsAfter implementation of the infarction protocol, the percentage of patients admitted with ST-segment elevation acute coronary syndrome and the mean GRACE score increased among patients admitted with acute coronary syndrome. Hospital stay was reduced, and primary angioplasty use increased. In-hospital mortality was reduced in high-risk patients, and prognosis after discharge was the same in both periods.
Keywords
Percutaneous coronary revascularization is the mainstay of treatment for acute coronary syndrome (ACS),1 particularly for ST-segment ACS (STEACS).2 The widespread use of coronary revascularization has led to the creation of more catheterization units and local and regional STEACS emergency care systems.3 These initiatives have been demonstrated to improve reperfusion rates and times.4–11 Most of the publications analyzing outcomes of an infarction protocol have focused purely on patients with STEACS4–7,9,11; however, this represents less than 35% of all patients with ACS.12,13
Over the past decade, the incidence of STEACS has decreased, while the incidence of non—ST-segment elevation ACS (NSTEACS) has remained steady or even increased.12,14,15 In NSTEACS, although invasive treatment has been demonstrated to be superior to conservative treatment,16,17 the revascularization rate is usually lower than in STEACS, and the patients usually have somewhat different clinical and hemodynamic profiles.12 The creation and implementation of an infarction protocol regulates emergency care only, almost always in relation to STEACS only. Nonetheless, it is easy to glean that the use of a common protocol that is standardized between different departments and hospitals could lead to an overall improvement in the treatment of patients with STEACS as well as those with NSTEACS.13,18,19 However, this has not been analyzed until now, and all the available evidence relates only to STEACS. The aim of our study was to describe the differences in clinical profile, treatment, and prognosis of patients with any type of ACS admitted to a secondary hospital with a cardiac catheterization laboratory after the implementation of a regional infarction protocol.
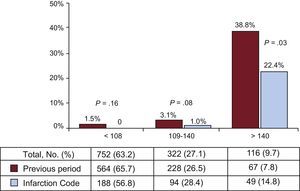
METHODSThis was an observational study of all patients admitted with ACS in the Hospital Universitario de San Juan in Alicante, in 2 defined periods: the 2 years prior to and the first year after implementation of the infarction protocol. The registry of patients with ACS and the informed consent form were approved by the hospital ethics committee. Acute coronary syndrome was defined as elevated enzyme markers of myocardial damage to above the normal limit of our hospital laboratory (troponin I ≥ 0.04 ng/dL or highly sensitive troponin > 0.056 ng/dL) and/or electrocardiographic changes indicative of myocardial ischemia or damage, with associated chest pain consistent with ACS.1 Patients were categorized according to GRACE score (Global Registry of Acute Coronary Events) into low-risk (< 108), intermediate-risk (109-140) and high-risk (> 140).20
During each admission, a record was made of the diagnosis, medical history, cardiovascular risk factors, treatments, investigations, and in-hospital complications of each patient. Glomerular filtration rate was estimated from serum creatinine levels using the Modification of Diet in Renal Disease Study equation.21 Statin therapy was considered intensive at a dose of 40 mg to 80 mg/day of atorvastatin and 20 mg to 40 mg/day of rosuvastatin, in line with the classification of the 2013 American guidelines on dyslipidemia.22 Combined analysis of comorbidities was performed using a modified Charlson index for patients with ischemic heart disease.23
Patients were followed up over the first year postdischarge by review of clinical notes and computerized medical records (from both primary care and the emergency department) and by telephone. The primary prognostic endpoint during follow-up was cardiovascular mortality, and the secondary endpoints were all-cause mortality, reinfarction, and the incidence of major cardiovascular complications (reinfarction, unplanned urgent revascularization, and readmission due to heart failure or stroke).
Statistical AnalysisAnalysis was carried out using the IBM program SPSS 22.0 for Mac. Qualitative variables were assessed using the chi-square test and Fisher's exact test when necessary; quantitative variables were compared using Student's t test and ANOVA. Factors associated with in-hospital mortality were identified using logistic regression, and the model included risk factors, history of cardiovascular disease, treatment received during hospital stay, and revascularization. Calibration of the logistic regression model was analyzed with the Hosmer-Lemeshow test, and diagnostic capacity was analyzed using the area below the ROC curve of probability estimated by the model. Survival analysis was performed using Cox proportional hazards regression, with forward stepwise selection, which included age, sex, all risk factors, any existing cardiovascular disease, treatment at discharge, and coronary revascularization. P-values < .05 were considered statistically significant.
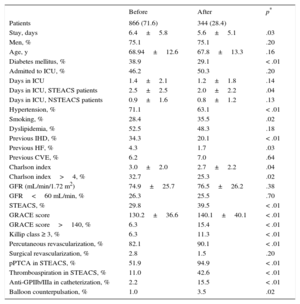
RESULTSDuring the study period, we included 1210 patients with a diagnosis of ACS. As shown in Table 1, the patients’ medical histories differed in the 2 periods: although the mean age was the same in both periods, patients admitted after implementation of the infarction protocol had a lower prevalence of diabetes mellitus and hypertension but a higher rate of active smoking. In addition, they generally had fewer comorbidities, as reflected by the lower Charlson index. After implementation of the infarction protocol, the percentage of patients admitted with STEACS and the mean GRACE score increased. Overall, percutaneous coronary revascularization significantly increased, and primary percutaneous transluminal coronary angioplasty became practically standard in STEACS. In the previous period, 21.3% of patients with STEACS were treated with thrombolysis and early percutaneous transluminal coronary angioplasty, but after implementation of the infarction protocol, this strategy was completely abandoned. In addition, the use of glycoprotein IIb/IIIa inhibitors, balloon counterpulsation, and thromboaspiration increased in patients treated after implementation of the infarction protocol.
General and Procedural Characteristics of Patients Before and After Implementation of the Infarction Code
| Before | After | p* | |
|---|---|---|---|
| Patients | 866 (71.6) | 344 (28.4) | |
| Stay, days | 6.4±5.8 | 5.6±5.1 | .03 |
| Men, % | 75.1 | 75.1 | .20 |
| Age, y | 68.94±12.6 | 67.8±13.3 | .16 |
| Diabetes mellitus, % | 38.9 | 29.1 | < .01 |
| Admitted to ICU, % | 46.2 | 50.3 | .20 |
| Days in ICU | 1.4±2.1 | 1.2±1.8 | .14 |
| Days in ICU, STEACS patients | 2.5±2.5 | 2.0±2.2 | .04 |
| Days in ICU, NSTEACS patients | 0.9±1.6 | 0.8±1.2 | .13 |
| Hypertension, % | 71.1 | 63.1 | < .01 |
| Smoking, % | 28.4 | 35.5 | .02 |
| Dyslipidemia, % | 52.5 | 48.3 | .18 |
| Previous IHD, % | 34.3 | 20.1 | < .01 |
| Previous HF, % | 4.3 | 1.7 | .03 |
| Previous CVE, % | 6.2 | 7.0 | .64 |
| Charlson index | 3.0±2.0 | 2.7±2.2 | .04 |
| Charlson index>4, % | 32.7 | 25.3 | .02 |
| GFR (mL/min/1.72 m2) | 74.9±25.7 | 76.5±26.2 | .38 |
| GFR<60 mL/min, % | 26.3 | 25.5 | .70 |
| STEACS, % | 29.8 | 39.5 | < .01 |
| GRACE score | 130.2±36.6 | 140.1±40.1 | < .01 |
| GRACE score>140, % | 6.3 | 15.4 | < .01 |
| Killip class ≥ 3, % | 6.3 | 11.3 | < .01 |
| Percutaneous revascularization, % | 82.1 | 90.1 | < .01 |
| Surgical revascularization, % | 2.8 | 1.5 | .20 |
| pPTCA in STEACS, % | 51.9 | 94.9 | < .01 |
| Thromboaspiration in STEACS, % | 11.0 | 42.6 | < .01 |
| Anti-GPIIb/IIIa in catheterization, % | 2.2 | 15.5 | < .01 |
| Balloon counterpulsation, % | 1.0 | 3.5 | .02 |
CVE, cerebrovascular event; GFR, glomerular filtration rate; GPIIb/IIIa, glycoprotein IIb/IIIa; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; ICU, intensive care unit; IHD, ischemic heart disease; NSTEACS, non—ST-segment elevation acute coronary syndrome; pPTCA, primary percutaneous transcatheter coronary angioplasty; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
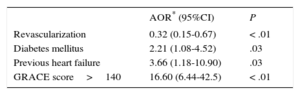
After implementation, time to catheterization was reduced (from 1.8 ± 2.5 hours to 1.0 ± 1.7 hours; P < .01), leading to an increase in patients receiving revascularization within 48 hours, from 65.4% to 78.6% (P < .01). These changes were due to patients admitted with STEACS: in patients with NSTEACS, there was no change in revascularization times or rates. Mean hospital stay was significantly shorter after implementation of the infarction protocol, mainly due to the reduction in hospital stay observed in patients with STEACS (7.0 ± 5.1 days vs 5.9 ± 4.5 days; P < .03). The overall rate of admission to the intensive care unit was similar in both periods; however, in patients admitted with STEACS, the length of stay in the unit was reduced after implementation of the infarction protocol. There were no differences in total in-hospital mortality between the 2 periods (4.9% vs 3.8%; P = .42), but in patients admitted with NSTEACS, there was a trend toward lower mortality after protocol implementation (3.9% vs 1.1%; P = .05); in patients with STEACS, in-hospital mortality was similar in both periods (7.4% vs 8.1%; P = .80). When patients were analyzed by GRACE score category, high-risk patients showed a reduction in in-hospital mortality after protocol implementation (Figure 1). As shown in Table 2, the independent variables associated with in-hospital mortality were diabetes mellitus, history of heart failure, and high-risk GRACE score; coronary revascularization was negatively associated.
Variables Associated With In-hospital Mortality
| AOR* (95%CI) | P | |
|---|---|---|
| Revascularization | 0.32 (0.15-0.67) | < .01 |
| Diabetes mellitus | 2.21 (1.08-4.52) | .03 |
| Previous heart failure | 3.66 (1.18-10.90) | .03 |
| GRACE score>140 | 16.60 (6.44-42.5) | < .01 |
95%CI, 95% confidence interval; GRACE, Global Registry of Acute Coronary Events; AOR, adjusted odds ratio.
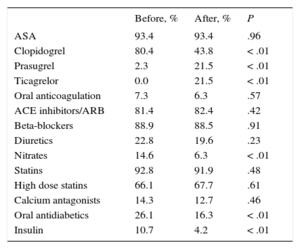
Table 3 shows the changes in the drugs prescribed at the time of discharge in each period: there were significant differences, particularly in the decreased use of clopidogrel and increased use of antiplatelet agents.
Treatment at Discharge Before and After Implementation of the Infarction Code
| Before, % | After, % | P | |
|---|---|---|---|
| ASA | 93.4 | 93.4 | .96 |
| Clopidogrel | 80.4 | 43.8 | < .01 |
| Prasugrel | 2.3 | 21.5 | < .01 |
| Ticagrelor | 0.0 | 21.5 | < .01 |
| Oral anticoagulation | 7.3 | 6.3 | .57 |
| ACE inhibitors/ARB | 81.4 | 82.4 | .42 |
| Beta-blockers | 88.9 | 88.5 | .91 |
| Diuretics | 22.8 | 19.6 | .23 |
| Nitrates | 14.6 | 6.3 | < .01 |
| Statins | 92.8 | 91.9 | .48 |
| High dose statins | 66.1 | 67.7 | .61 |
| Calcium antagonists | 14.3 | 12.7 | .46 |
| Oral antidiabetics | 26.1 | 16.3 | < .01 |
| Insulin | 10.7 | 4.2 | < .01 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid.
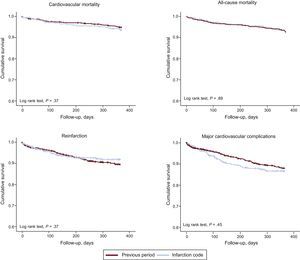
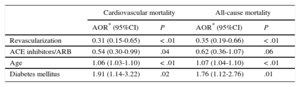
Follow-up over the first year was achieved in 93.1% of patients discharged in each period, with a mean follow-up of 370 days [interquartile range, 359-382 days] and of equal length in both periods. There were 1154 patients discharged from hospital, with 82 (7.1%) recorded deaths during follow-up, of which 61 (5.3%) were of cardiovascular cause. As shown in Figure 2, there were no differences in cardiovascular mortality (5.0% vs 6.0%), all-cause mortality (7.3% vs 6.6%), reinfarction (9.3% vs 7.0%) or major cardiovascular complications (10.9% vs 11.7%) in comparison with the period before the infarction protocol. On multivariate analysis, no association was found between mortality during follow-up and implementation of the infarction protocol (Table 4); an association was observed between revascularization and reduced mortality.
Variables Associated With Cardiovascular Mortality or All-cause Mortality During Follow-up
| Cardiovascular mortality | All-cause mortality | |||
|---|---|---|---|---|
| AOR* (95%CI) | P | AOR* (95%CI) | P | |
| Revascularization | 0.31 (0.15-0.65) | < .01 | 0.35 (0.19-0.66) | < .01 |
| ACE inhibitors/ARB | 0.54 (0.30-0.99) | .04 | 0.62 (0.36-1.07) | .06 |
| Age | 1.06 (1.03-1.10) | < .01 | 1.07 (1.04-1.10) | < .01 |
| Diabetes mellitus | 1.91 (1.14-3.22) | .02 | 1.76 (1.12-2.76) | .01 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; AOR, adjusted odds ratio; ARB, angiotensin receptor blockers.
The introduction of an infarction protocol in our hospital led to changes in the overall clinical profile of patients admitted with ACS, with an increased mean GRACE score and increased percentage of patients admitted with STEACS, and an overall increase in the use of revascularization. Following implementation of the infarction protocol, although no differences were observed in mortality in the first year postdischarge, in-hospital mortality was reduced in high-risk patients, and, strikingly, in patients with NSTEACS. In addition, hospital stay was reduced by 1 day, despite the patients admitted in the second period being more unwell. Most publications on the subject that have analyzed the effect of implementing an infarction protocol have focused solely on patients with STEACS4–7,9,11; however, patients with STEACS make up less than 35% of all patients with ACS.12,13 Therefore, the analysis performed in this study may be regarded as more comprehensive and representative of everyday clinical practice: it compares all patients admitted with ACS over the first year postdischarge after implementation of the infarction code with those admitted in the previous 2 years. We also consider our outcomes to be representative of everyday clinical practice because the general characteristics, GRACE score, and incidence of complications in the patients included were similar to those of other registries.4–13,18 Furthermore, the study conforms to the recommendations of the European Society of Cardiology that the creation of a new infarction care plan should be accompanied by a prospective registry that evaluates outcomes.3
The most immediate consequence of implementing an infarction protocol in a hospital with a cardiac catheterization laboratory is that primary angioplasty becomes the standard reperfusion strategy for STEACS. This would explain the increased use of revascularization in patients with ACS observed in the second period. The increased percentage of patients admitted with a diagnosis of STEACS may be due to various reasons: although a higher incidence of this type of ACS cannot be ruled out, a more obvious explanation would be that patients with an indication for primary angioplasty were no longer transferred to the old referral hospital with a 24 hour on-call cardiac catheterization service. However, most of these transferred patients would subsequently return to our hospital, except for a minority of cases whose clinical situation did not allow a return. Another reason could have been increased diagnostic sensitivity. The implementation of the protocol was accompanied by a strong training and refresher campaign on the diagnostic and therapeutic criteria with the different departments involved, following the recommendations of the European Society of Cardiology.3 Diagnoses that were previously uncertain–in the presence of inconclusive electrocardiograms such as left bundle branch block or paced rhythms, atypical symptoms, or out-of-hospital cardiac arrests–were recognized as STEACS in the second period and received the appropriate diagnosis and treatment. In our system, the on-call interventional cardiologist was often consulted in cases of doubt and contributed to improved diagnostic accuracy in such cases. Gómez-Hospital et al6 analyzed the impact of a local infarction protocol in the southern metropolitan area of Barcelona, finding that patients had a lower risk profile after implementation of the protocol. That study, unlike ours, included only patients referred for primary or rescue angioplasty. Their results may reflect that in the phase prior to implementation, mechanical reperfusion was indicated only for the most unwell patients, and the criteria were more restrictive, for example patients with contraindications for thrombolysis.6 More recently, the DICOCLES12 registry showed that almost 20% of patients with STEACS received no reperfusion treatment, and primary angioplasty was used for only 2 thirds of reperfusion cases. In our study, patients who were severely unwell and, possibly, patients with more difficult diagnoses were correctly identified as STEACS and included in the protocol. Immediate mechanical reperfusion contributes to the early diagnosis of multivessel disease, to reduced in-hospital complications, and to more effective management of these patients.24
Urgent transfer to catheterization units for revascularization of STEACS has been demonstrated to be safe and effective.4,5,11 This has led to the increased use of local and/or regional systems for STEACS care, known as infarction codes, but can also lead to significant treatment delays.5,9 Based on their outcomes, these care systems have been demonstrated to improve times6,7,11 and overall reperfusion rates4,7,9; however, very few programs have been able to demonstrate significant reductions in mortality.4–7,9 In the DIOCLES registry,12 74% of patients with STEACS were taken to hospital by ambulance, whereas in patients with NSTEACS, this figure was only 55.4%. Our results generally coincide with this datum and, furthermore, show a reduction in in-hospital mortality in high-risk patients. Finally, the pharmacoinvasive strategy, not currently considered a first option, ceased to be used in our hospital, although this option has been demonstrated to be similar to primary angioplasty in terms of left ventricular recovery and mortality.25
Mortality in the first year after discharge was the same in both periods. This finding may seem disheartening due to the effort involved in the implementation and continuation of an infarction code program; however, taking into account that patients in the second period had a higher mean GRACE score and higher percentage of STEACS, the mere fact that they did not have higher mortality could be considered positive. In fact, the crude mortality rate was slightly higher among patients from the period with the infarction code, although the difference was not statistically significant. These findings correspond entirely with those published in other national7 and international8,10 registries. It has been argued that this finding is due to the inclusion of increasingly complex patients and more critical situations, such as resuscitated out-of-hospital cardiac arrests or patients with increased delay times who would not have been included in initial emergency STEACS care protocols.10 An analysis of outcomes following implementation of an infarction code in the Barcelona metropolitan area showed a reduction in mortality and major cardiovascular complications at 1 year.6 However, as already mentioned, that study included only patients treated with percutaneous reperfusion; therefore, unlike our study, those results may reflect an effort to improve transfer times and patient care,6 which is invariably associated with improved prognosis,9,10 as well as a more widespread use of the technique without limitation to only the most severe cases.
LimitationsThe main limitations of our study relate to it being an observational study in a single center. However, the sample size was larger than that of other national publications assessing the outcomes of regional plans,4–7,11 and follow-up was for 1 year after discharge. The technical and pharmacological resources and their recommendations in clinical practice guidelines may have changed between the 2 periods2,26,27; however, the medical staff was unchanged during the 2 periods and the working method did not change except for the organization of the infarction code program. In addition, given that not all the medication administered during admission was recorded, it cannot be excluded that there were differences in the pharmacological management between the 2 periods, although it is likely that these differences would be minimal. Lastly, the study was unable to analyze the differences in costs derived from the implementation of the infarction code; while it is true that the mean hospital stay was reduced, it cannot be excluded that there was an increased use of materials, human resources, and logistics that could counteract this saving.
CONCLUSIONSAfter implementation of the infarction protocol in a secondary hospital with a cardiac catheterization unit, the percentage of patients admitted with STEACS and the mean GRACE score increased among patients admitted with ACS. Overall in-hospital mortality was unchanged, but prognosis improved in high-risk patients, and a there was a trend toward improved mortality in patients with NSTEACS. As a reperfusion method, primary angioplasty became standard, and hospital stay was significantly reduced. However, prognosis in the first year postdischarge from hospital was the same before and after the implementation of the protocol. Our results show that the implementation of the infarction code led to improved organization and revascularization in all patients with ACS, not only those with STEACS. Increased use of primary angioplasty requires coordination between the different departments involved in the emergency care of STEACS, and our data show that the implementation of an infarction protocol resulted in improved ACS treatment in general, but particularly for high-risk patients and/or those with STEACS. The data from this study support the need for implementation of such systems in hospitals that are not integrated in local or regional plans.
CONFLICTS OF INTERESTNone declared.