Myocarditis is defined as an inflammatory disease of the heart muscle and is an important cause of acute heart failure, sudden death, and dilated cardiomyopathy. Viruses account for most cases of myocarditis or inflammatory cardiomyopathy, which could induce an immune response causing inflammation even when the pathogen has been cleared. Other etiologic agents responsible for myocarditis include drugs, toxic substances, or autoimmune conditions. In the last few years, advances in noninvasive techniques such as cardiac magnetic resonance have been very useful in supporting diagnosis of myocarditis, but toxic, infectious-inflammatory, infiltrative, or autoimmune processes occur at a cellular level and only endomyocardial biopsy can establish the nature of the etiological agent. Furthermore, after the generalization of immunohistochemical and viral genome detection techniques, endomyocardial biopsy provides a definitive etiological diagnosis that can lead to specific treatments such as antiviral or immunosuppressive therapy. Endomyocardial biopsy is not commonly performed for the diagnosis of myocarditis due to safety reasons, but both right- and left endomyocardial biopsies have very low complication rates when performed by experienced operators. This document provides a state-of-the-art review of myocarditis and inflammatory cardiomyopathy, with special focus on the role of endomyocardial biopsy to establish specific treatments.
Keywords
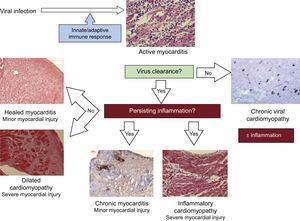
The term myocarditis refers to an inflammation of the heart muscle, which can be caused by infections, toxic substances, or autoimmune processes. During the acute phase, a specific trigger induces an immune response, which can range from transient and mild to fulminant. In the case of viral myocarditis, if the host does not success in eliminating the infectious pathogen, chronic infection develops, with or without ongoing inflammation. Furthermore, inflammation can persist even if the pathogen has been cleared. Thus, inflammatory dilated cardiomyopathy (DCM) is an independent entity with its own pathogenic mechanisms and a potential cause of heart failure. As understanding of this disease increases, it is now evident that the pathological injury occurs at the cellular level, and therefore an accurate diagnosis requires tissue analysis with endomyocardial biopsy (EMB).1 Histological findings have been proved to have prognostic implications,2 and in several cases specific treatments can be added to the basic symptomatic heart failure treatment.3 T this review aims to serve as a practical document for the diagnosis and treatment of myocarditis and inflammatory cardiomyopathy, with special focus on EMB as a diagnostic tool, as well as on subsequent tailored treatment based on its results.
DEFINITIONS AND ETIOLOGY OF MYOCARDITISMyocarditis is defined by an inflammation of the myocardium diagnosed by established histological, immunological, and immunohistochemical criteria. As stated in the consensus paper of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases,1 it is histologically defined by the presence of inflammatory infiltrates in the myocardium associated with myocyte degeneration and necrosis of nonischemic cause, following the Dallas criteria.4 Regarding immunohistochemical criteria, the aforementioned document proposes that diagnosis should be made attending to the presence of at least 14 leukocytes/mm2 in the myocardium including up to 4 monocytes/mm2 and with detection of 7 or more CD3-positive T lymphocytes.1 As for inflammatory DCM, the World Health Organization/International Society and Federation of Cardiology defines it as myocarditis in association with cardiac dysfunction.1
Myocarditis and inflammatory cardiomyopathies can be caused by infections, drugs, toxic substances, and autoimmune diseases (Table 1). Infectious agents are the most common etiologic factors, with viral infections being the leading cause of acquired inflammatory cardiomyopathies in Europe and North America.2
Etiology of Inflammatory Cardiomyopathy
| Infectious | Noninfectious |
|---|---|
| Virus | Autoimmune |
| Adenoviruses | Post-infectious |
| Enteroviruses (Coxsackie A/B, echovirus) | Influenza vaccination |
| Cytomegalovirus | Systemic lupus erythematosus |
| Erythroviruses | Sarcoidosis |
| Herpesviruses | Sjögren syndrome |
| Influenza A/B | Churg-Strauss syndrome |
| HIV | Wegener granulomatosis |
| Hepatitis virus C | Takayasu arteritis |
| Poliovirus | Inflammatory bowel disorders |
| Varicella zoster | Giant cell myocarditis |
| Arboviruses | |
| Mixed infections | |
| Bacteria | Toxins |
| Mycobacteria | Anthracyclines |
| Chlamydia | Catecholamines |
| Streptococci | Cytokines |
| Mycoplasma | Cocaine |
| Legionella spp. | Alcohol |
| Salmonella spp. | Chemotherapeutic drugs |
| Rickettsia spp. | |
| Corynebacteria | |
| Borrelia spp. | |
| Fungi | Allergic/hypersensitive |
| Aspergillus | Penicillin |
| Candida | Tricyclic antidepressants |
| Cryptococus | Clozapine |
| Histoplasmodium spp. | Antirheumatic drugs |
| Sulfonamides | |
| Cephalosporins | |
| Parasites and protozoa | Physical pathogens |
| Schistosomiasis | Arsenic |
| Larva migrans | Lithium |
| Trypanosoma cruzi | Irradiation |
| Toxoplasma gondii | Hypothermia |
| Trichinosis/trichinellosis | Heat stroke |
| Echinococci |
Other rare causes of myocarditis are systemic autoimmune diseases such as Loeffler disease or Churg-Strauss syndrome, which can be associated with eosinophilic myocarditis. Furthermore, cardiac sarcoidosis and giant cell myocarditis represent other infrequent cases in which early diagnosis and treatment initiation are crucial, as they will determine prognosis.
Viral MyocarditisDue to the routine use of molecular biology methods, the spectrum of cardiotropic viruses has greatly expanded. The most common isolated genotypes are those from enteroviruses, adenovirus, parvovirus B19 (PVB19) (which belongs to the erythrovirus family), herpes virus type 6 (HHV-6), Epstein-Barr virus and cytomegalovirus (the latter particularly in immunocompromised patients). About 30% of patients have multiple-agent infections of the myocardium.5 The epidemiology of myocardial virus species changes depending on the geographical sites, but in the last decade erythrovirus and herpes virus genomes have been detected more frequently than enterovirus or adenovirus,6 contrary to what has been previously described. Such a high prevalence of erythrovirus and herpes virus may be due to a high incidence of childhood infection and their subsequent lifelong persistence.7 Thus, their detection in different tissues can indicate a latent infection and symptoms may appear due to reactivation.3 Moreover, not all viruses cause myocarditis with the same patterns of infection. For instance, enteroviruses and adenoviruses directly infect cardiomyocytes in animals and humans and in the last few years, 10% to 15% of viral myocarditis have been caused by these agents.8
The situation of erythroviruses such as PVB19 is quite different. This virus primarily infects erythroid progenitor cells in the bone marrow9 and endothelial cells, leading to asymptomatic and latent infections. Then, when the virus becomes reactivated, angina-like symptoms have been related to endothelial dysfunction.10
CLINICAL SYMPTOMS OF MYOCARDITIS AND NONINVASIVE DIAGNOSISThe clinical presentation of myocarditis varies widely, ranging from ischemic-like chest pain to syncope or acute heart failure. Although most patients present with mild symptoms or transient electrocardiographic changes, myocarditis can also cause acute heart failure and life-threatening cardiogenic shock.1
It frequently starts 1 to 4 weeks after an infection, normally respiratory or gastrointestinal. However, due to its varied symptoms, myocarditis can be difficult to diagnose, and coronary artery disease must always be excluded, given its high prevalence and similar clinical presentation. Moreover, EMB is undoubtedly the gold standard diagnostic tool in myocarditis and inflammatory cardiomyopathy. No other test can provide a definite diagnosis, and noninvasive techniques are used to help clinicians rule out other diagnoses and indirectly recognize myocarditis.
ElectrocardiogramAll patients with suspected myocarditis should receive a 12-lead electrocardiogram.1 Electrocardiographic findings in myocarditis patients include T-wave and ST-segment changes, ST-segment elevation mimicking acute myocardial infarction or conduction abnormalities (as seen in Lyme disease, cardiac sarcoidosis, or giant cell myocarditis).11 These changes are nonspecific and can be found in other clinical settings, but the electrocardiogram is still an easily available screening tool. Regarding prognosis, prolonged QRS duration of > 120ms is the only independent factor for heart transplantation or cardiac death.12
Imaging TechniquesEchocardiography remains the key method for analyzing ventricular function in suspected myocarditis and helps to rule out other entities such as valve disease. Thus, all patients with suspected myocarditis should undergo echocardiographic studies at presentation and during follow-up.1 However, findings are nonspecific, and include global ventricular dysfunction, regional wall motion abnormalities, or diastolic dysfunction. Both in acute and fulminant myocarditis, wall thickness may be mildly increased, but left ventricular (LV) diastolic dimensions are typically larger in acute myocarditis. As for systolic function, better recovery is normally seen in patients that survive after the acute phase of fulminant myocarditis when compared with acute myocarditis.13 In fact, it has been observed that fulminant myocarditis may have a good outcome in severe clinical settings such as Dengue when specific treatment is applied.14 Regarding patients with preserved left ventricular ejection fraction (LVEF), speckle tracking is a promising tool. In patients with biopsy-proven myocardial inflammation, global longitudinal strain rate and global longitudinal strain are significantly impaired compared with patients without inflammation, regardless of conventional echocardiographic parameters15 Therefore, this technique has a higher sensitivity in the detection of mild myocardial damage in patients with preserved LVEF and plays a role in predicting outcome, as patients with impaired baseline strain show worse follow-up echocardiographic results.
Cardiac magnetic resonance (CMR) can help confirm the diagnosis of myocarditis, especially in the acute phase of the disease. The combined use of 3 different CMR techniques is suggested, and findings are compatible with myocardial inflammation if at least 2 Lake Louise criteria are met.
These include: a) Regional or global myocardial signal intensity increase in T2-weighted edema images; b) an increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images, and c) at least 1 focal lesion with nonischemic regional distribution in inversion recovery-prepared gadolinium-enhanced T1-weighted images.16 When at least 2 criteria are met, a sensitivity of 76% and specificity of 96% have been reported in patients with clinically suspected acute myocarditis and pseudo-infarction presentation.17
Moreover, recent studies have shown good correlations between CMR results and EMB in acute myocarditis, with up to 79% accuracy when new CMR techniques are used.18 However, obtaining the biopsy from the region of late gadolinium enhancement of the CMR has not proven to increase the yield of diagnosis19 and, in chronic myocarditis, the diagnostic performance of CMR was found to be worse (sensitivity, 63%; specificity, 40%).16 Therefore, CMR might not be appropriate to guide clinical management in chronic myocarditis.
BiomarkersCardiac troponins are highly suggestive of acute myocarditis, when other potential causes of myocardial necrosis, such as acute coronary syndromes, have been excluded.20 Elevation of cardiac troponin I or T levels is more common than creatine kinase MB and persistent high levels indicate ongoing necrosis. NT-pro-BNP or BNP levels should be measured when heart failure is suspected, but normal values do not exclude myocarditis.21 Newer cardiac biomarkers, such as copeptin or midregional pro-adrenomedullin, do not provide additional diagnostic or prognostic information.20
The usefulness of viral serologies is limited, especially in chronic myocarditis or inflammatory cardiomyopathy, as IgG antibodies for cardiotropic virus can be found in the blood stream of the general population without accompanying cardiac involvement.22 A positive virus polymerase chain reaction (PCR) in peripheral blood does not prove viral myocarditis either. However, when viral genome is present in EMB, blood viral PCR can exclude or confirm systemic infection.2 It may also allow the discrimination of an acute viral infection from endogenous viral reactivation, in which there is higher virus replication.
Regarding serum cardiac autoantibodies (anti fibrillary, organ-specific and partially organ-specific antiheart, anti-intercalated disks, anti-interfibrillary, etc.), these can be useful when high levels are present in the absence of viral genome in EMB, suggesting an immune mediated myocarditis or inflammatory cardiomyopathy.
ENDOMYOCARDIAL BIOPSYEndomyocardial biopsy is the gold standard technique for the diagnosis of myocarditis and inflammatory cardiomyopathy. The toxic, infectious-inflammatory, infiltrative or autoimmune processes that cause myocarditis occur at a cellular level, and no other diagnostic techniques can establish the nature of the etiological agent. As well as detection of inflammation or viral genomes in the acute phase of myocarditis, EMB adds important prognostic information during the follow-up of patients that can influence therapeutic decisions. The 2007 American Heart Association/American College of Cardiology Foundation/European Society of Cardiology scientific statement on EMB limited its class I recommendations to unexplained new-onset heart failure of less than 2 weeks’ duration associated with hemodynamic compromise or unexplained new-onset heart failure of 2 weeks to 3 months’ duration associated with a dilated LV and new ventricular arrhythmias or conduction disturbances.23 However, in a recent position statement from the European Society of Cardiology,1 the recommendation for EMB was extended, including patients with a pseudo-infarct presentation after exclusion of coronary artery disease. This change reflects the generalization of immunohistochemical and viral genome detection techniques, which have enabled progress in the etiological diagnosis of myocarditis. Hence, an increasing number of patients can benefit from specific treatments.
The main reason for the restriction of EMB procedures in some centers is safety. Nonetheless, when performed by experienced operators, both right and left EMB have very low complication rates. In a single-center study that analyzed 3048 EMB in a nontransplant setting, the risk of major complications including cardiac tamponade and atrioventricular block requiring permanent pacemaker implantation was 0.12%. No deaths were registered.24 Previous studies also reported a major complications rate of less than 0.5%.25 Left ventricular biopsy has also been proven to be a safe procedure.26 Chimenti et al26 documented that over a 28-year period and over 4000 EMB, complications appeared in only 0.33% of patients who underwent left EMB.
How to Perform an Endomyocardial BiopsyEndomyocardial biopsy is performed with the patient in a supine position under local anesthesia with 2% lidocaine. The patient must be monitored with 3-lead electrocardiogram, noninvasive blood pressure monitoring, and oxygen saturation. An international normalized ratio of < 1.5 is required before the EMB, and anticoagulation therapy should be discontinued 16hours before and 12hours after the procedure. Vascular access for right ventricular (RV) EMB is usually through the femoral or right internal jugular vein. Left ventricular or biventricular EMB is preferred through the right femoral vein and femoral artery for access to the RV and LV.27 The bioptomes used are warranted to be flexible in order to ensure safety. We recommend the modified Cordis bioptomes. This bioptome (B-18110; Medizintechnik Meiners, Monheim, Germany) has been used in clinical practice since 1985. It has a 6 Fr diameter and a length of 1100mm. Compared with the conventional Cordis bioptome, it has a more flexible polytetrafluoroethylene (Teflon) tube.
Endomyocardial biopsy should be guided by fluoroscopy to locate the intraventricular septum in case of RV EMB, as the thinness of the free wall lead to a high perforation risk. When it comes to LV EMB, one of the main concerns is potential severe mitral regurgitation due to biopsied chordae, so fluoroscopy can also help to prevent this situation. Moreover, an echocardiogram is recommended before and after the procedure to exclude pericardial effusion.
A recent study has evaluated the feasibility and safety of LV EMB via transradial access with promising results,28 offering a less invasive alternative to the classic femoral approach, and could reduce hospital stays.
With reference to the number of samples taken by the procedure, we recommend at least 5 and up to 10 to guarantee reliable results. Focal tissue involvement is frequent in myocarditis and so different parts of the RV septum or the LV should be biopsied. Samples for histology and immunohistochemical analysis should be at least 1-2mm and promptly fixed in 10% formalin or snap frozen in liquid nitrogen depending on the antibody that is going to be used. Samples for virus genome analysis should be snap frozen in liquid nitrogen, stored at −80°C, or stored in RNAlater® tubes at room temperature.1
Left Ventricular vs Right Ventricular Endomyocardial BiopsyThe diagnostic value of LV vs RV EMB has been analyzed in various studies, and the results are not homogeneous. Whereas some observe that the diagnostic yield of LV EMB is superior to that of RV EMB when routine immunohistochemistry and viral genome amplification are used in suspected LV myocarditis,26 more recent data indicate that both procedures are similar when inflammation or viral genome are being assessed in the myocardium. However, in this latter study, morphological changes such as interstitial fibrosis and cardiac collagen type I expression were more reliably found in LV EMB.29
Interpretation of Endomyocardial Biopsy Results and Prognostic ImplicationsIn all suspected cases of myocarditis, tissue samples from LV or RV should be analyzed using histology, immunohistochemistry, and viral genomes (viral PCR in EMB and blood).1 All of these features help us to diagnose inflammation and the presence of viral genome, which have prognostic implications and require specific treatments.
InflammationThe histology of inflammation in the myocardium was originally defined by the qualitative Dallas criteria (presence of inflammatory infiltrates in the myocardium associated with myocyte degeneration and necrosis of nonischemic cause).
Later, the addition of immunohistochemical criteria with different monoclonal antibodies increased the EMB sensitivity in the diagnosis of myocarditis30 and inflammation was quantitatively established at ≥ 14 leucocytes /mm2. During EMB analysis, specific inflammatory cells can be distinguished by cluster differentiation (CD). B-cells are CD20 positive and all T cells are CD3 positive. T cell subpopulations include CD4 (helper), CD8 (suppressor) and CD45R0 (memory or activated T-cells) or perforin-positive cytotoxic lymphocytes. CD68 and CD11 stand for macrophages. Attending these subpopulations, inflammation can be more specifically diagnosed by > 7.0 CD3+ lymphocytes/mm2 and/or > 35.0 CD11b+/Mac-1+ macrophages/mm2.31
During an acute inflammatory disease course, the histology or immunohistology samples normally contain focal or diffuse cell infiltration by lymphocytes and/or macrophages. Other cells such as eosinophils or giant cells are rare.
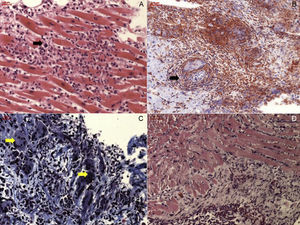
Active lymphocytic myocarditis is characterized by acute cell necrosis in addition to the aforementioned infiltrates, contrary to borderline myocarditis that does not show necrosis (Figure 1). Other acute entities such as idiopathic eosinophilic myocarditis, giant cell myocarditis, granulomatous disorders, and allergic-induced types of myocarditis are rare and are found in less than 20% of cases.8 Regarding prognostic predictors in the acute phase, it has been observed that the density of inflammatory cell infiltrate in the acute phase determines long-term disease course.32 Moreover, prognosis changes, depending on the cell infiltrate characteristics. Thus, borderline focal myocarditis has an excellent prognosis, whereas the early mortality of fulminant lymphocytic myocarditis is 40% in the first month.32 Outcome is even worse in untreated eosinophilic or giant cell myocarditis, in which survival is less than 20% after 4 years.33,34
Different types of acute myocarditis. A: Acute lymphocitic myocarditis with focal inflammatory cell infiltrates (black arrow) and cardiomyocite necrosis. B: Cardiac sarcoidosis, with evidence of granuloma (black arrow). C: Giant cell myocarditis, with presence of giant multinucleated cells (yellow arrows). D: Eosinophilic myocarditis.
Regarding patients with DCM and chronic heart failure, inflammation is seen in up to 30% of biopsies.8 However, cells tend to distribute in a more diffuse manner than in the acute phase and other features are present on histological examination, such as hypertrophy of the cardiomyocytes and interstitial fibrosis (Figure 2).
Lately, immunohistological signs of inflammation have been also related to poor outcome in suspected myocarditis. Actually, positive immunohistology for invading immune cells and expression of HLA-DR-α, but not the Dallas criteria alone, was associated with a higher risk of cardiac death or heart transplantation in patients with acute and chronic myocarditis.35 In a more recent study that excluded patients with acute myocarditis, perforin was a predictor of LVEF course in patients with chronic inflammatory cardiomyopathy and negative genomes for cardiotropic virus (enteroviruses, adenoviruses, Epstein-Barr virus, HHV-6). Erythrovirus was present in 54% of patients, but without evidence of transcriptional activity.31 Even though all patients received recommended heart failure treatment during follow-up, a perforin value of more than 2.95 was associated with LVEF deterioration (94.2% sensitivity and 80.4% specificity).31
Presence of Viral GenomeIn western countries, most of the infectious agents causing myocarditis are viruses, and the viral spectrum differs with geographical location.8 The PCR identifies viral DNA or RNA in the myocardium with very high sensitivity.1 Firstly, nested PCR identifies the virus qualitatively, and if positive, viral load is measured by real-time PCR.
All samples should be compared with negative controls and controlled by amplifying adequate positive samples1 and latent infections can be differentiated from acute cases with parallel analyses of the blood stream.
Among the viral agents causing myocarditis, it is important to distinguish 2 groups: newly acquired infections and endogenous virus infections with subsequent reactivations.
Enteroviruses and adenoviruses constitute the first group. They are established causes of acute myocarditis and can also be detected in DCM presenting as chronic heart failure.36 As previously stated, myocardial injury is caused by direct cardiomyocyte infection or antiviral immunity. After the acute infection, 60% to 70% of patients completely recover without residual injuries due to an efficient immunity that is able to clear the virus. Hence, a follow-up biopsy will reveal healed myocarditis. However, if the initial injury is already significant with important loss of contractile tissue and remodeling, patients do not completely recover even if the virus is cleared or inflammation disappears.
Various studies have evaluated the effect of enteroviral genome persistence.
While it is true that the effect of viral persistence on outcome is unclear in other virus species (PVB19 or HHV-6), mortality is higher in patients with noncleared enterovirus. Why et al37 observed 25% mortality at 25 months in myocarditis/DCM patients with persistent enteroviral infection, as opposed to 4% mortality in enterovirus-negative patients. Similar data have been more recently published, with a mortality as high as 41% in patients with enteroviral genome persistence after a 5-year follow-up.38
Regarding PVB19 and HHV-6 infections, the most common clinical entities are persistent latent virus infections with reactivation episodes.22 PVB19 is a common acute disease during childhood, rarely seen in adults. Basically, infected cells are limited to erythroid progenitors in the bone marrow, but the primary erythrovirus receptor is also present in endothelial cells, including the heart. Although it has been exceptionally localized in venuoles or arterioles during fulminant myocarditis in children,39 in most cases the infection is latent and asymptomatic. Recently, we have reported that about 30% of PVB19-positive EMB had messenger RNA, which may indicate reactivation of the virus.10 In this context, it has been observed that cardiac gene expression is altered. For instance, genes involved in inflammatory response (tissue necrosis factor alpha, related orphan receptor C) or mitochondrial energy metabolism (cyclooxygenase-1) are deregulated in messenger RNA-positive patients compared with those with only DNA.40 However, the effect of PVB19 DNA persistence on outcome is still not clear, as case series in which this virus was the most prevalent did not demonstrate a higher risk of death or high transplantation rates.35 Moreover, systolic dysfunction has not been clearly related to the presence of PVB19, but in a group of 37 patients with unexplained diastolic dysfunction, 84% were PVB19-positive in EMB, suggesting a relationship with the endothelial dysfunction caused by the virus.41
The HHV-6A and HHV-6B also usually cause acute infections during childhood, and like PVB19, remains latent in > 70% of adults. Although HHV-6 is mainly a lymphotropic virus, it can also infect both endothelial cells and cardiomyocytes. In addition, its genome can be integrated into human chromosomes and transmitted through the germ line.42 Similar to PVB19, HHV-6 can become reactivated causing heart failure symptoms, and a recent study suggests that HHV-6 persistence could lead to a worsening in LVEF and clearance to an improvement.43
Irrespective of the initial viral etiology, if a biopsy is performed when the patient is already in the chronic phase without evidence of a previous viral infection or persistent inflammation, the diagnosis will be idiopathic DCM.22 Other clinical scenarios are persistent lytic virus infection without inflammation (chronic viral heart disease), or continual autoimmune mechanisms even when the virus has been cleared (inflammatory cardiomyopathy). When both inflammation and viral infection persist, then the diagnosis is chronic viral cardiomyopathy.8 All these clinical entities are summarized in Figure 2.
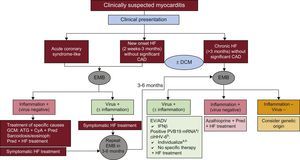
TREATMENT OF MYOCARDITIS AND INFLAMMATORY CARDIOMYOPATHYRegardless of its etiology, the basic treatment of myocarditis is the optimal care of heart failure and arrhythmias in accordance with evidence-based guidelines.44 Nonconventional and specific treatments depend on the result of the EMB, taking into account the patients’ symptoms and the disease course33,34,45 (Figure 3).
Myocarditis treatment according to clinical setting and endomyocardial biopsy results. ADV, adenovirus; ATG, anti thymoglobulin; CAD, coronary artery disease; ciHHV-6, chromosomally integrated human herpes virus type 6; CyA, cyclosporin; DCM, dilated cardiomyopathy; EV, enterovirus; EMB, endomyocardial byopsy; GCM, giant cell myocarditis; HF, heart failure; IFNβ, interferon beta; mRNA, messenger RNA; Pred, prednisone; PVB19, parvovirus B19. aIn symptomatic patients, consider interferon beta or other potential options under study such as telbivudine (see text). bIn symptomatic patients despite optimal heart failure treatment, consider ganciclovir or valganciclovir (see text).
Hemodynamically unstable patients should be managed in intensive care units with invasive monitoring and a skilled team of professionals for cardiac catheterization and the performance of EMB. In patients who develop progressive deterioration of cardiac pump function despite conventional treatment, EMB is essential to diagnose potentially treatable causes such as giant cell or eosinophilic myocarditis. However, as myocardial injuries progress rapidly and can quickly become irreversible, a mechanical cardio-pulmonary assist device or extracorporeal membrane oxygenation may sometimes be needed as a bridge to heart transplantation or recovery.1
Stable patients with systolic ventricular dysfunction should be treated with diuretics, renin angiotensin aldosterone system inhibitors, and beta-adrenergic blockade. The specific moment when these drugs should be withdrawn after LVEF recovery is not well defined.46 Regarding nonsteroidal anti-inflammatory drugs, their use is not recommended due to a mortality increase in animal experimental models of myocarditis, even though they are widely implemented in the treatment of pericarditis. Implantable cardioverter defibrillator implantation is only recommended if symptoms and systolic cardiac dysfunction persist after the acute phase. In the meantime, when a patient is discharged after acute myocarditis with low LVEF, wearable cardioverter defibrillators (LifeVest®) can provide protection as a bridge to implantable cardioverter defibrillator decision.
Specific Treatments During the Acute PhaseBiopsy-proven acute viral myocarditis often improves spontaneously in more than 60% of patients with conventional heart failure treatment and therefore close follow-up is usually sufficient in these patients.8 In fact, the initial cardiac inflammation helps to eliminate the virus as soon as possible to prevent irreversible myocardial injuries, and anti-inflammatory or immunosuppressive therapy can favor viral persistence and therefore worsen the patient's outcome.47 However, it is still not well studied whether the presence of specific markers such as perforin during acute myocarditis affect prognosis, and if these patients could benefit from early treatment.
On the other hand, other clinical entities benefit strongly from specific treatments during the acute phase. Combined treatment of giant cell myocarditis with antithymoglobulin, cyclosporine (through level 100-120μg/mL) and cortisone has proved to improve survival in previous studies34 (Table 2, treatment regimens).
Current Therapeutic Options in Acute Giant Cell Myocarditis and Autoimmune Inflammatory Cardiomyopathy
| Giant cell myocarditis (Cooper et al33,34) |
| Antithymoglobulin |
| 275mg in 500mL 0.9% saline solution for 12 h/24 h |
| Days 1 to 5 |
| Under cardiac monitoring |
| Ciclosporine |
| Start dose 200 mg/24 h (100 mg/12 h) |
| Targeted trough level: 100-120μg/mL |
| 1 year |
| Methylprednisolone |
| Initial dose: 1 mg/kg |
| After 4 weeks: decrease by 10mg, and then another 10mg every 2 weeks until 5-10mg maintenance dose |
| 1 year |
| Cardiac sarcoidosis |
| Methylprednisolone |
| Initial dose: 1 mg/kg |
| After 4 weeks: decrease by 10mg, and then another 10mg every 2 weeks until 5-10mg maintenance dose |
| 6 months |
| Chronic/autoimmune myocarditis (inflammatory cardiomyopathy), eosinophilic myocarditis (Frustaci et al45) |
| Azathioprine |
| 50 mg/12 h for 6 months |
| Weekly laboratory control with blood count/liver enzymes during the first month |
| Contemplate other alternatives if < 3000 leucocytes or < 1000 lymphocytes |
| Methylprednisolone |
| Initial dose: 1 mg/kg |
| After 4 weeks: decrease by 10mg, and then another 10mg every 3 weeks until 5-10mg maintenance dose |
| 6 months |
| Accompanying treatment in all cases pantoprazole/omeprazole 20 mg/24 h, calcium 1 g/24 h |
Hypereosinophilic syndrome, or Loeffler disease, usually develops in 3 stages. In the acute phase, mature eosinophils infiltrate and damage the myocardium and hypereosinophilia is evidenced in peripheral blood. Then, valve involvement and apical obliteration are observed and the final stage is endomyocardial fibrosis.48 During the acute phase, in which extensive irreversible fibrosis is not present, antihelminthic or antiprotozoal drugs can be used in the tropical form of the disease. In all other clinical scenarios, immunosuppression is recommended. The most common treatment regimen is cortisone and azathioprine, with cortisone being decreased every 2 weeks by 10mg from an initial dose of 1mg/kg until a maintenance dose of 10mg for 6 months. Other therapeutic options that have proved some benefit in this entity include interferon (IFN) or tyrosine-kinase inhibitors (Imatinib).49
Granulomatous acute myocarditis is sometimes seen in cardiac sarcoidosis or rheumatoid arthritis. Prednisone alone is a good option in these cases with an initial dose of 1mg/kg, although other immunosuppressive drugs such as azathioprine can be added (Table 2). A long treatment of at least 6 months is warranted.
Specific Treatments in Chronic Inflammatory CardiomyopathiesAutoimmune/Virus NegativeIn some patients, inflammation persists, despite viral clearance, as evidenced in follow-up EMB 6 months after the initial onset of disease. In these patients, the inflammatory process is due to a post-infectious state or autoimmunity. Some randomized trials have shown that immunosuppressive therapy in these patients is superior to conventional treatment alone in terms of LVEF and New York Heart Association classification improvement.45 In the TIMIC study, chronic myocarditis virus-negative patients with less than 45% LVEF who received conventional heart failure for at least 6 months were randomized to placebo vs cortisone and azathioprine.45 The LVEF improved in 89% of patients from the treatment group and in none of the placebo group. Furthermore, a previous study observed that only virus-negative patients improved with immunosuppression.47
Because circulating autoantibodies have been detected in DCM patients, they may play a role as markers of autoimmunity in clinical and biopsy-proven myocarditis.50 On this basis, immunoadsortion may be a treatment option in the future. Limited randomized studies have shown that this therapy improves LVEF,51 and some have highlighted the role of specific markers, such as β-1 adrenoceptor antibodies, whose clearance with immunoadsortion leads to longer heart transplant-free survival.51 However, larger investigations are warranted and currently immunoadsortion is still an experimental therapy.
Viral CardiomyopathyAs previously stated, some viruses infect cardiomyocytes directly, such as enterovirus or adenovirus, and others, such as PVB19 or HHV-6, damage endothelial cells. Thus, treatment schemes and response vary depending on the species.
Among patients with chronic enteroviral or adenoviral cardiomyopathy, viral clearance with a 6-month course of IFNβ therapy was accompanied by LVEF improvement and a significant decrease of ventricular dimensions in a nonrandomized trial.52 After a 5-year follow-up, 92% of patients who had cleared the virus were alive compared with only 69% of patients with virus persistence.38
Furthermore, it was observed that patients who cleared the virus spontaneously had higher levels of endogenous IFNβ than those with viral persistence. Thus, these findings support the efficacy of IFNβ therapy.
In patients infected by PVB19, it is very important to differentiate between latent infection (with positive DNA alone) and viral reactivations (with positive messenger RNA as well). In fact, 1 study observed that B19V messenger RNA was only present in myocardial biopsy samples from patients with inflammation and was absent in B19V DNA-positive patients without inflammation.53 Regarding specific treatment regimens, IFNβ does not eliminate the virus. However, a study with PVB19 observed that endothelial dysfunction and secondary symptoms improved with high doses of IFNβ, suggesting that this treatment could inhibit PVB19 reactivation and improve endothelium viability.54 Other potential treatment options are still under study. For instance, the thymidine analog telbivudine suppresses viral replication in vitro and, in a pilot trail with 8 PVB19-positive and symptomatic patients, a 6-month course of treatment with this drug silenced transcriptional activity in 7 out of 8 patients and improved symptoms within the first weeks.
The HHV-6 is also not cleared by IFNβ, but a recent study observed a decrease in HHV-6 reactivation after a 6-month course of treatment with valganciclovir in symptomatic patients with reactivated (messenger RNA-positive) chromosomally integrated HHV-6 and unexplained symptoms of heart failure. Symptoms also improved with the treatment.55 For the moment, only those patients with reactivated chromosomally integrated HHV-6 apparently benefit from antiviral treatment, but it should be used as an alternative option in patients with persistent symptoms despite conventional treatment.
Specific doses and treatments for each of the viruses are summarized in Table 3.
Current Therapeutic Options for Viral Cardiomyopathies
| Enteroviral/adenoviral cardiomyopathy (Kuhl et al38) |
| Interferon beta |
| 4 million units subcutaneously every 48 h for the first week |
| 8 million units subcutaneously every 48 h from the second week and for 6 months |
| Follow-up laboratory tests 2 weeks after initiation (including Cr, liver enzymes, blood count, TSH/T3/T4, cTnT/cTnI, CK/CK-MB, then monthly |
| Stop treatment if < 100 000 platelets or < 2000 leucocytes |
| Messenger RNA positive PVB19 cardiomyopathy (Bock et al,53Schmidt-Lucke et al54) |
| Interferon beta |
| 4 million units subcutaneously every 48 h for the first week |
| 8 million units subcutaneously every 48 h from the second week and for 6 months |
| Other potential therapeutic options under study: telbivudine |
| Symptomatic HHV-6 reactivations (Pellett et al42, Escher et al43) |
| Ganciclovir 1000 mg/24 h intravenously 5 days |
| Then: valganciclovir 900 mg/ 24 h or 1800 mg/24 h |
| • For 6 months |
| • Follow-up laboratory tests 2 weeks after initiation (including Cr, liver enzymes, blood count), then monthly |
| • Stop treatment if: neutropenia, anemia or hepatitis |
Cr, creatinine; CK, creatine kinase; CK-MB, creatine kinase MB isoenzyme; cTnI, cardiac troponin I; cTnT, cardiac troponin T; HHV-6: human herpes virus type 6; PVB19, parvovirus B19; T3, triiodothyronine; T4, thyroxine; TSH: thyroid stimulating hormone.
High-dose intravenous immunoglobulins have been used in chronic symptomatic heart failure of different etiologies and have been associated with improved LVEF,56 but a major controlled trial showed no benefit in recent-onset DCM.57 The lack of improvement was probably due to the fact that only 16% of the patients had inflammation in the EMB and viral genomes were not analyzed. However, there are currently no specific recommendations for the use of intravenous immunoglobulins in myocarditis.1
CONCLUSIONSMyocarditis is a cardiac inflammatory disease mainly caused by viral infections or autoimmune processes. Despite the advances of noninvasive diagnostic tests, especially in the CMR field, EMB remains the gold standard diagnostic technique for myocarditis and inflammatory cardiomyopathy. After acute myocarditis, the inflammatory process is spontaneously resolved in most patients after 1 to 4 months. However, sometimes the immune response fails to eliminate the infectious agent and the inflammatory process does not resolve, causing damage to the myocardium. In these settings, EMB can elucidate the cause of the disease and specific treatments can be initiated in addition to standard antifailure therapy if myocardial injury is still not irreversible. Other conditions such as giant cell myocarditis or cardiac sarcoidosis benefit from treatment during the acute phase and therefore EMB plays an important role in both acute and chronic settings. Despite the promising results with immunosuppressive or antiviral therapy in specific clinical scenarios according to published data, larger randomized studies are warranted to detect the effect of these treatments on strong clinical endpoints such as heart transplantation or mortality.
CONFLICTS OF INTERESTNone declared.