Transcatheter aortic valve implantation is increasingly used in patients with aortic stenosis. Post-procedural intraventricular conduction abnormalities and permanent pacemaker implantations remain a serious concern. Recently, the Edwards SAPIEN 3 prosthesis has replaced the SAPIEN XT. We sought to determine the incidences of new-onset intraventricular conduction abnormalities and permanent pacemaker implantations by comparing the 2 devices.
MethodsWe analyzed the last consecutive 103 patients undergoing transcatheter aortic valve implantation with SAPIEN XT before SAPIEN 3 was used in the next 105 patients. To analyze permanent pacemaker implantations and new-onset intraventricular conduction abnormalities, patients with these conditions at baseline were excluded. Electrocardiograms were recorded at baseline, after the procedure, and before discharge.
ResultsSAPIEN 3 was associated with higher device success (100% vs 92%; P=.005) and less paravalvular leakage (0% vs 7%; P<.001). The incidence of permanent pacemaker implantations was 12.6% (23 of 183) with no difference between the 2 groups (SAPIEN 3: 12.5% [12 of 96] vs SAPIEN XT: 12.6% [11 of 87]; P=.99). SAPIEN 3 was associated with a higher rate of new-onset intraventricular conduction abnormalities (49% vs 27%; P=.007) due to a higher rate of fascicular blocks (17% vs 5%; P=.021). There was no statistically significant difference in transient (29% [20 of 69] vs persistent 19% [12 of 64]; P=.168) left bundle branch blocks (28% [19 of 69] vs 17% [11 of 64]; P=.154) when SAPIEN 3 was compared with SAPIEN XT.
ConclusionsWe found a trend toward a higher rate of new-onset intraventricular conduction abnormalities with SAPIEN 3 compared with SAPIEN XT, although this did not result in a higher permanent pacemaker implantation rate.
Keywords
Transcatheter aortic valve implantation (TAVI) is increasingly used as a therapeutic strategy in high-risk patients with severe symptomatic aortic stenosis and has shown encouraging clinical results in large registries1,2 and randomized trials.3,4 However, post-procedural intraventricular conduction abnormalities (IVCA) and the need for permanent pacemaker implantation remain a serious concern.
New-onset IVCA, especially new-onset left bundle branch block (LBBB), are observed in a considerable proportion of patients after TAVI (between 8% and 30%)5,6 with a balloon-expandable valve7–9 and may negatively affect recovery of left ventricular function and increase the risk for permanent pacemaker implantation.10,11
Furthermore, the occurrence of high-grade atrioventricular conduction abnormalities leading to permanent pacemaker implantation is a frequent complication associated with TAVI, with an incidence ranging between 3% and 11.5% with balloon-expandable valves.3,12
Recently, a new generation balloon expandable prosthesis, Edwards SAPIEN 3 (Edwards Lifesciences; Irvine, CA, United States)13 has been introduced replacing the SAPIEN XT in Germany. The SAPIEN 3 device has a higher metal frame and features an outer skirt surrounding the valve's frame, designed to avoid paravalvular leakage. The incidence of new-onset IVCA and rates of permanent pacemaker implantation with this novel prosthesis are still under investigation. Ideally, the improved hemodynamics of novel devices should not be associated with a higher rate of conduction abnormalities.
This study assessed the incidence of new-onset IVCA and the need for permanent pacemaker implantation with the SAPIEN 3 prosthesis in comparison with the SAPIEN XT valve in a consecutive cohort of patients undergoing TAVI.
METHODSPatient Populations and Transcatheter Aortic Valve Implantation ProcedureWe prospectively included 222 patients undergoing TAVI at the Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München during a 12-month period (SAPIEN XT (n=103) from July 2nd 2013 until February 11th 2014 and SAPIEN 3 (n=105) from January 31st to June 26th 2014). Patients receiving other valve types were not included (n=14).
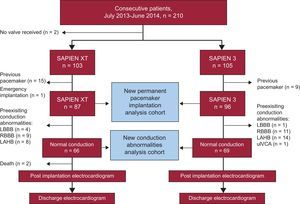
To determine the incidence of new permanent pacemaker implantation after TAVI, patients carrying a pacemaker (n=24) as well as 1 patient who underwent emergency TAVI were excluded. Additionally, to analyze the incidence of new-onset IVCA after TAVI, patients with IVCA at baseline were excluded (n=48) (Figure 1).
Study design and flow of patients. In total, 210 patients were suitable for transfemoral TAVI using an Edwards valve during the prespecified observation interval. Of these, 103 and 105 patients received SAPIEN XT and SAPIEN 3 valves, respectively. Two patients did not undergo TAVI due to inadequate access site (n=1) and short distance to the coronary ostium (n=1). After exclusion of patients with a prior permanent pacemaker, and 1 patient who underwent transcatheter aortic valve implantation in an emergency situation, 183 patients were available for new permanent pacemaker implantation analysis comparing the 2 devices. New-onset IVCA were analyzed in patients with normal electrocardiograms at baseline (n=135). LAHB, left anterior hemiblock; LBBB, left bundle branch block; RBBB, right bundle branch block; uIVCA, unspecific intraventricular conduction abnormality.
TAVI was performed in a hybrid operating room using the transfemoral approach in all patients.
Multislice Computed Tomography and Prosthesis Size SelectionAortic annulus measurements were assessed in multiple plane reconstruction according to the guidelines of the Society of Cardiovascular Computed Tomography.14 The area and perimeter of the virtual aortic annulus were obtained by direct planimetry. The minimal and maximum diameters were determined and the effective diameter was calculated as the sum of the minimum and maximum diameter divided by 2. The eccentricity of the aortic annulus was calculated as the eccentricity index using the formula [1-minimum diameter/maximum diameter]. The closer this index comes to zero, the more circular the aortic annulus. Dedicated Food and Drug Administration approved software (OsiriX MD 3.9.4, Pixmeo, Switzerland) was used. The respective prosthesis size was selected according to the manufacturers’ recommendations but the final decision on prosthesis size was left at the discretion of the physicians performing the procedure.
The SAPIEN XT and SAPIEN 3 heart valves are available in 23-, 26- and 29-mm sizes covering a nominal area of 4.15cm2, 5.31cm2, and 6.21cm2 and 4.06cm2, 5.19cm2, and 6.48cm2, respectively. The percentage of oversizing according to area was calculated using the formula (nominal prosthesis area/patient annular area-1)×100.
Electrocardiogram Analysis and Permanent Pacemaker ImplantationsElectrocardiograms were recorded at a minimum of 3 time points: on admission (at least 24hours before TAVI), immediately after TAVI, and before discharge. Electrocardiogram data were reviewed by 2 physicians blinded to clinical data according to current recommendations.15 Controversial cases were solved by consensus. The following electrocardiographic variables were evaluated at all time points: rhythm (sinus rhythm, atrial fibrillation or flutter), heart rate, PQ duration and QRS intervals, atrioventricular conduction disturbances (none, grade I, II and III), and IVCA (complete bundle branch block, including left and right bundle branch block separately), fascicular blocks (including left anterior and left posterior hemiblock and incomplete left and right bundle branch block), and nonspecific IVCA. New-onset IVCA, present after TAVI but not at discharge, were classified as transient, whereas IVCA still present at discharge were termed persistent.
The decision to implant a permanent pacemaker was left at the discretion of the physicians in charge of the patients’ treatment. The indications were recorded in every case. In patients with normal intraventricular conduction at baseline undergoing permanent pacemaker implantation after TAVI, the last available electrocardiogram prior to permanent pacemaker implantation was used for the analysis of new-onset IVCA.
Follow-up and Definition of OutcomesAll baseline, procedural, postoperative and electrocardiogram data were prospectively recorded. In-hospital outcome, device success and procedural complications were categorized using the updated criteria defined by the Valve Academic Research Consortium.16
Statistical AnalysisContinuous variables are expressed as mean±standard deviation or as median [interquartile range] and compared using the paired or unpaired Student t test or Mann-Whitney U test, as appropriate. Discrete variables were compared with the chi-square test or Fisher's exact test, as appropriate. To assess the influence of the learning curve with the novel device, the cohort of patients was divided into quartiles, and the incidence of new permanent pacemaker implantation and new persistent LBBB were analyzed. A 2-sided P-value of<.05 was considered statistically significant. SPSS 20 (IBM Corporation; United States) was used.
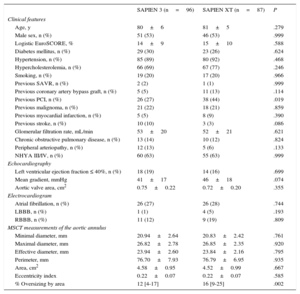
RESULTSBaseline CharacteristicsBaseline characteristics according to prosthesis type are shown in Table 1. There were no statistically significant differences between the 2 groups except a higher rate of previous percutaneous coronary intervention in the SAPIEN XT cohort (44% vs 27%; P=.019).
Baseline Characteristics of the SAPIEN 3 and SAPIEN XT Subgroups
| SAPIEN 3 (n=96) | SAPIEN XT (n=87) | P | |
|---|---|---|---|
| Clinical features | |||
| Age, y | 80±6 | 81±5 | .279 |
| Male sex, n (%) | 51 (53) | 46 (53) | .999 |
| Logistic EuroSCORE, % | 14±9 | 15±10 | .588 |
| Diabetes mellitus, n (%) | 29 (30) | 23 (26) | .624 |
| Hypertension, n (%) | 85 (89) | 80 (92) | .468 |
| Hypercholesterolemia, n (%) | 66 (69) | 67 (77) | .246 |
| Smoking, n (%) | 19 (20) | 17 (20) | .966 |
| Previous SAVR, n (%) | 2 (2) | 1 (1) | .999 |
| Previous coronary artery bypass graft, n (%) | 5 (5) | 11 (13) | .114 |
| Previous PCI, n (%) | 26 (27) | 38 (44) | .019 |
| Previous malignoma, n (%) | 21 (22) | 18 (21) | .859 |
| Previous myocardial infarction, n (%) | 5 (5) | 8 (9) | .390 |
| Previous stroke, n (%) | 10 (10) | 3 (3) | .086 |
| Glomerular filtration rate, mL/min | 53±20 | 52±21 | .621 |
| Chronic obstructive pulmonary disease, n (%) | 13 (14) | 10 (12) | .824 |
| Peripheral arteriopathy, n (%) | 12 (13) | 5 (6) | .133 |
| NHYA III/IV, n (%) | 60 (63) | 55 (63) | .999 |
| Echocardiography | |||
| Left ventricular ejection fraction ≤ 40%, n (%) | 18 (19) | 14 (16) | .699 |
| Mean gradient, mmHg | 41±17 | 46±18 | .074 |
| Aortic valve area, cm2 | 0.75±0.22 | 0.72±0.20 | .355 |
| Electrocardiogram | |||
| Atrial fibrillation, n (%) | 26 (27) | 26 (28) | .744 |
| LBBB, n (%) | 1 (1) | 4 (5) | .193 |
| RBBB, n (%) | 11 (12) | 9 (19) | .809 |
| MSCT measurements of the aortic annulus | |||
| Minimal diameter, mm | 20.94±2.64 | 20.83±2.42 | .761 |
| Maximal diameter, mm | 26.82±2.78 | 26.85±2.35 | .920 |
| Effective diameter, mm | 23.94±2.60 | 23.84±2.16 | .795 |
| Perimeter, mm | 76.70±7.93 | 76.79±6.95 | .935 |
| Area, cm2 | 4.58±0.95 | 4.52±0.99 | .667 |
| Eccentricity index | 0.22±0.07 | 0.22±0.07 | .585 |
| % Oversizing by area | 12 [4-17] | 16 [9-25] | .002 |
LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MSCT, multislice computer tomography; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; SAVR, surgical aortic valve replacement.
Diabetes mellitus: insulin-dependent as well as noninsulin dependent diabetes mellitus. Hypertension: resting blood pressure > 140/90mmHg and/or medication with antihypertensive agents. Hypercholesterolemia: total cholesterol > 200mg/dL and/or medication with cholesterol synthesis inhibitors. Smoking: present and past smokers. Atrial fibrillation denotes rhythm on admission electrocardiogram.
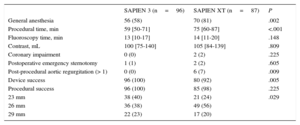
Significantly more procedures were performed under conscious sedation using the SAPIEN 3 device. Use of the SAPIEN 3 valve was associated with a significantly higher device success rate (100% vs 92%; P=.005) and a lower prevalence of higher than grade 1 post-procedural aortic regurgitation (0% vs 7%; P=.009). Procedural time was significantly shorter for patients treated with the SAPIEN 3 prosthesis compared with its predecessor (59 vs 75minutes; P<.001) (Table 2). While aortic annulus measurements on multislice computer tomography did not differ between the 2 groups, oversizing by area was significantly larger in the case of SAPIEN XT compared with SAPIEN 3 (16% vs 12%; P=.002) (Table 1).
Procedural Data of the SAPIEN 3 and SAPIEN XT Cohorts
| SAPIEN 3 (n=96) | SAPIEN XT (n=87) | P | |
|---|---|---|---|
| General anesthesia | 56 (58) | 70 (81) | .002 |
| Procedural time, min | 59 [50-71] | 75 [60-87] | <.001 |
| Fluoroscopy time, min | 13 [10-17] | 14 [11-20] | .148 |
| Contrast, mL | 100 [75-140] | 105 [84-139] | .809 |
| Coronary impairment | 0 (0) | 2 (2) | .225 |
| Postoperative emergency sternotomy | 1 (1) | 2 (2) | .605 |
| Post-procedural aortic regurgitation (> 1) | 0 (0) | 6 (7) | .009 |
| Device success | 96 (100) | 80 (92) | .005 |
| Procedural success | 96 (100) | 85 (98) | .225 |
| 23 mm | 38 (40) | 21 (24) | .029 |
| 26 mm | 36 (38) | 49 (56) | |
| 29 mm | 22 (23) | 17 (20) |
Data are expressed as no. (%), or median [interquartile range].
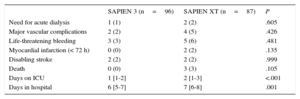
Patients treated with SAPIEN 3 had a significantly shorter stay in the intensive care unit (1 day vs 2 days; P<.001) and in hospital (6 days vs 7 days; P<.001). There was no statistically significant difference in in-hospital events between the 2 groups (Table 3).
In-hospital Events of the SAPIEN 3 and SAPIEN XT Subgroups
| SAPIEN 3 (n=96) | SAPIEN XT (n=87) | P | |
|---|---|---|---|
| Need for acute dialysis | 1 (1) | 2 (2) | .605 |
| Major vascular complications | 2 (2) | 4 (5) | .426 |
| Life-threatening bleeding | 3 (3) | 5 (6) | .481 |
| Myocardial infarction (< 72 h) | 0 (0) | 2 (2) | .135 |
| Disabling stroke | 2 (2) | 2 (2) | .999 |
| Death | 0 (0) | 3 (3) | .105 |
| Days on ICU | 1 [1-2] | 2 [1-3] | <.001 |
| Days in hospital | 6 [5-7] | 7 [6-8] | .001 |
ICU, intensive care unit.
Data are expressed as no. (%), or median [interquartile range].
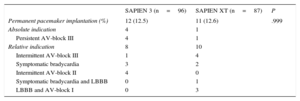
The incidence of permanent pacemaker implantation after TAVI was 12.5% in the SAPIEN 3 (12 of 96) and 12.6% the SAPIEN XT groups (11 of 87). The baseline characteristics of patients with and without permanent pacemaker implantation after TAVI are depicted in the The reasons for permanent pacemaker implantation after TAVI are displayed in Table 4. There were no significant differences between the 2 groups regarding relative and absolute indications for permanent pacemaker implantation. Patients requiring permanent pacemaker implantation had less normal conduction (10 of 23 [44%] vs 125 of 160 [78%]; P<.001), more right bundle branch block (8 of 23 [35%] vs 12 of 160 [8%]; P<.001) and a longer QRS interval (113±26ms vs 99±20ms; P=.023) than patients not requiring permanent pacemaker implantation. Multislice computer tomography data and the percentage of oversizing by area did not differ between patients requiring permanent pacemaker implantation and those who did not ().
Incidence and Reasons for Permanent Pacemaker Implantations After Transcatheter Aortic Valve Implantation
| SAPIEN 3 (n=96) | SAPIEN XT (n=87) | P | |
|---|---|---|---|
| Permanent pacemaker implantation (%) | 12 (12.5) | 11 (12.6) | .999 |
| Absolute indication | 4 | 1 | |
| Persistent AV-block III | 4 | 1 | |
| Relative indication | 8 | 10 | |
| Intermittent AV-block III | 1 | 4 | |
| Symptomatic bradycardia | 3 | 2 | |
| Intermittent AV-block II | 4 | 0 | |
| Symptomatic bradycardia and LBBB | 0 | 1 | |
| LBBB and AV-block I | 0 | 3 |
AV-block, atrioventricular block; LBBB, left bundle branch block.
The incidence and reasons for permanent pacemaker implantations after transcatheter aortic valve implantation in both cohorts was determined after exclusion of patients carrying a pacemaker (n=24) at baseline.
The rate of permanent pacemaker implantation in the SAPIEN 3 cohort was stable across consecutive tertiles, indicating no decrease of permanent pacemaker implantation rate with growing experience using the new device (P for trend .803).
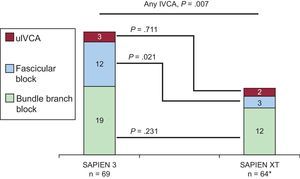
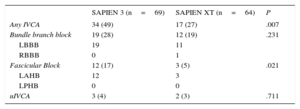
New-onset Intraventricular Conduction AbnormalitiesIn total, 135 patients without IVCA in the baseline electrocardiogram underwent TAVI, of whom 66 and 69 were treated with the SAPIEN XT and the SAPIEN 3 valve, respectively. Follow-up electrocardiogram data were not available in 2 patients treated with the SAPIEN XT prosthesis due to intraprocedural death. New-onset IVCA before discharge with the 2 devices are depicted in Figure 2 and Table 5. Overall, patients treated with the SAPIEN 3 prosthesis had a significantly lower rate of normal intraventricular conduction (51% vs 73%; P=.007) mainly due to a significantly higher rate of new left anterior hemiblock (17% vs 5%; P=.021). The incidence of new-onset bundle branch block did not differ between the 2 devices.
New-onset intraventricular conduction abnormalities with SAPIEN 3 compared with SAPIEN XT. Absolute numbers for the respective IVCA are displayed in the graphs. Thirty-five (51%) and 47 (73%) patients in the SAPIEN 3 and SAPIEN XT cohorts did not display new-onset IVCA at discharge (P=.007), respectively (see Table 5 for details). IVCA, intraventricular conduction abnormality; uIVCA, unspecific intraventricular conduction abnormality.
* In 2 patients, no discharge electrocardiographic data were available due to intraprocedural death.
New-onset Intraventricular Conduction Abnormalities According to SAPIEN 3 and SAPIEN XT at Discharge
| SAPIEN 3 (n=69) | SAPIEN XT (n=64) | P | |
|---|---|---|---|
| Any IVCA | 34 (49) | 17 (27) | .007 |
| Bundle branch block | 19 (28) | 12 (19) | .231 |
| LBBB | 19 | 11 | |
| RBBB | 0 | 1 | |
| Fascicular Block | 12 (17) | 3 (5) | .021 |
| LAHB | 12 | 3 | |
| LPHB | 0 | 0 | |
| uIVCA | 3 (4) | 2 (3) | .711 |
IVCA, intraventricular conduction abnormality; LAHB, left anterior hemiblock; LBBB, left bundle branch block; LPHB, left posterior hemiblock; RBBB, right bundle branch block; IVCA, intraventricular conduction abnormality; uIVCA, unspecific intraventricular conduction abnormalities.
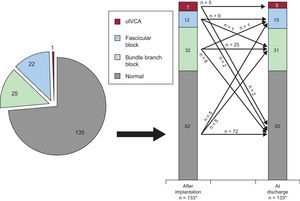
The trend in new-onset IVCA from the post-procedural phase to discharge in the entire population is depicted in Figure 3. New transient LBBB and persistent LBBB occurred in 24% (32 of 133) and 22% (30 of 133) of patients, respectively. There was a trend towards a higher number in the occurrence of transient (29% [20 of 69] vs 19% [12 of 64]; P=.168) and persistent LBBB (28% [19 of 69] vs 17% [11 of 64]; P=.154) in SAPIEN 3 compared with SAPIEN XT patients, although there was no statistically significant difference. Data stratified into SAPIEN 3 and SAPIEN XT treated patients are displayed in the
Trend in new-onset intraventricular conduction abnormalities after transcatheter aortic valve implantation in the entire study population. For details see text. Absolute numbers for the respective IVCA are displayed in the graphs. IVCA, intraventricular conduction abnormality; uIVCA, unspecific intraventricular conduction abnormality.
* In 2 patients, no discharge electrocardiographic data were available due to intraprocedural death.
To analyze whether persistent new-onset LBBB was affected by the experience with the new device, we looked at the tertiles of consecutive patients, but observed a stable rate in the SAPIEN 3 cohort with time (P for trend .876).
DISCUSSIONRecently, the Edwards SAPIEN 3 device replaced the SAPIEN XT valve in Europe. The present study reports our initial experience with this novel device and focuses on the incidence of new-onset IVCA and permanent pacemaker implantation in comparison with its predecessor. We found a trend towards a higher rate of new-onset IVCA in the SAPIEN 3 cohort while permanent pacemaker implantation rates were similar compared with SAPIEN XT.
Permanent Pacemaker Implantations After Transcatheter Aortic Valve ImplantationThe incidence of permanent pacemaker implantation with the SAPIEN XT valve ranges between 5% and 12%.3,17 In our patients, the permanent pacemaker implantation rate with the SAPIEN XT valve was similar to these previous observations. Our analysis also confirmed that pre-existing right bundle branch block was associated with permanent pacemaker implantation after TAVI.18,19
Pacemaker dependency may limit clinical benefit from TAVI due to lack of atrioventricular-synchrony and right ventricular pacing,20 although some authors did not observe an effect of permanent pacemaker implantation on clinical outcome in terms of survival.12,21 Additionally, permanent pacemaker implantation after TAVI has been identified as an important cause of prolonged hospital stay, thereby increasing procedural costs.22 Thus, the need for permanent pacemaker implantation as a specific aspect of clinical outcome after TAVI is of major importance.
In the present study, we report the permanent pacemaker implantation rate using the novel SAPIEN 3 device. Interestingly, despite a trend toward a higher rate of new-onset IVCA, use of the SAPIEN 3 device did not result in a higher rate of permanent pacemaker implantation during hospitalization compared with its predecessor, SAPIEN XT. As far as pacemaker implantations after TAVI with the SAPIEN 3 is concerned, our data are in line with emerging data indicating a rate of 13% to 30%.23–25
The specific indications for permanent pacemaker implantation after TAVI are frequently not reported in the literature. While there are absolute indications, eg, persistent complete atrioventricular block, several clinical scenarios lead to relative indications. To date, no consensus has been reached for clear permanent pacemaker implantation indications after TAVI. Current recommendations include electrocardiographic monitoring of TAVI patients and acknowledge that there is a proportion of patients at higher risk who may require prolonged monitoring.26 There is also emerging evidence that atrioventricular-conduction abnormalities after TAVI may resolve over time. Indeed, one study reported resolution of perioperative atrioventricular block in more than half of the patients after implantation of a self-expandable valve.27 Indications for permanent pacemaker implantation in the present study were left at the discretion of the physician in charge of the patients’ treatment. We report a considerable number of patients with a relative indication for permanent pacemaker implantation. Due to the elevated risk for events in the high-risk TAVI population our, policy for permanent pacemaker implantation was more liberal.
New-onset Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. Incidence and Clinical ImpactNew-onset LBBB is a frequent observation after TAVI with balloon-expandable valves7–9 and is regarded as a direct result of procedure-induced injury to the conduction system. Indeed, most conduction abnormalities already occur during valvuloplasty28 and a tendency to reversal in a small proportion of patients has been reported.6 In the present study, we found that 22% of LBBB occurring after transcatheter aortic valve implantation resolved before discharge. However, 6 patients with no LBBB after TAVI developed LBBB before discharge so the absolute number of complete LBBB at discharge did not differ significantly.
The clinical relevance of LBBB after TAVI remains a subject of ongoing investigations. While no impact on mortality has been observed, there is evidence that new-onset LBBB after transcatheter aortic valve implantation may be associated with a higher risk of late permanent pacemaker implantation and impaired recovery of left ventricular function.6,8,10,11
The incidence of new-onset LBBB after TAVI with the SAPIEN XT valve ranges between 8% and 30%.5,6 Our current study reports the incidence of new-onset IVCA with a nonsignificant trend toward a higher incidence of new-onset persistent LBBB of 28% using the SAPIEN 3 device compared with 17% in the SAPIEN XT group. Additionally, there was a significantly increased rate of overall new-onset IVCA in the SAPIEN 3 group.
The potential underlying mechanisms for these findings may be due to differences in prosthesis design and implantation mechanisms. SAPIEN 3 features a higher metal frame than SAPIEN XT and an outer skirt to minimize paravalvular regurgitation. Differences in valve deployment with more foreshortening of SAPIEN 3 within the left ventricular outflow tract during implantation may lead to increased mechanical trauma to the cardiac conduction system, which may potentially explain the higher incidence of fascicular blocks observed in the present study. Another relevant factor may be the height of implantation within the aortic annulus, which was not analyzed in the present study. Lastly, the appropriateness of sizing algorithms issued by the manufacturer need to be confirmed. Future research should also focus on the identification of the underlying pathophysiological mechanisms.
Strengths and LimitationsThe present study is limited by its sample size and is not based on a randomized comparison of the 2 devices. The findings of our study are strengthened by the fact that all procedures were performed by the same team in a highly experienced TAVI center and that rates of IVCA reported with the SAPIEN XT device are similar to previously published data. However, the influence of a learning curve in the SAPIEN 3 group may not be completely ruled out due to previous experience with SAPIEN XT. In addition, although new-onset LBBB was increased by 58% in the SAPIEN 3 cohort compared with our SAPIEN XT cohort, the observed incidence of 28% is still in the range reported previously for the SAPIEN XT valve.5,6 Accordingly, LBBB rates with the SAPIEN 3 in other centers may vary considerably.
The depth of implantation has been identified as a predictor of new conduction abnormalities.6,29,30 Unfortunately, this parameter was not available for all patients in the present study.
Although the in-hospital rate of new permanent pacemaker implantations did not differ, a possible influence of the higher rate of new-onset conduction abnormalities on the incidence of late permanent pacemaker implantation with SAPIEN 3 remains to be determined by larger studies with long-term follow-up.
CONCLUSIONSIn our initial experience, we found a trend toward a higher rate of new-onset IVCA with SAPIEN 3; however, we observed a similar rate of permanent pacemaker implantation compared with its predecessor, the SAPIEN XT prosthesis. Further research focusing on potential underlying mechanisms and possible strategies to reduce the incidence of new-onset IVCA is warranted.
CONFLICTS OF INTERESTC. Hengstenberg and A.M. Kasel are proctors for Edwards Lifesciences.