The study of myocardial mechanics has a potential role in the detection of cardiac involvement in patients with amyloidosis. This study aimed to characterize 3-dimensional-speckle tracking echocardiography-derived left and right ventricular myocardial mechanics in light chain amyloidosis and examine their relationship with brain natriuretic peptide.
MethodsIn patients with light chain amyloidosis, left ventricular longitudinal and circumferential strain (n=40), and right ventricular longitudinal strain and radial displacement (n=26) were obtained by 3-dimensional-speckle tracking echocardiography. Brain natriuretic peptide levels were determined.
ResultsAll myocardial mechanics measurements showed differences when compared by brain natriuretic peptide level tertiles. Left and right ventricular longitudinal strain were highly correlated (r=0.95, P<.001). Left ventricular longitudinal and circumferential strain were reduced in patients with cardiac involvement (–9±4 vs –16±2; P<.001, and –24±6 vs –29±4; P=.01, respectively), with the most prominent impairment at the basal segments. Right ventricular longitudinal strain and radial displacement were diminished in patients with cardiac involvement (–9±3 vs –17±3; P<.001, and 2.7±0.8 vs 3.8±0.3; P=.002). On multivariate analysis, left ventricular longitudinal strain was associated with the presence of cardiac involvement (odds ratio = 1.6; 95% confidence interval, 1.04 to 2.37; P=.03) independent of the presence of brain natriuretic peptide and troponin I criteria for cardiac amyloidosis.
ConclusionsThree-dimensional-speckle tracking echocardiography-derived left and right ventricular myocardial mechanics are increasingly altered as brain natriuretic peptide increases in light chain amyloidosis. There appears to be a strong association between left ventricular longitudinal strain and cardiac involvement, beyond biomarkers such as brain natriuretic peptide and troponin I.
Keywords
Systemic amyloidosis in its light chain (AL) variant is characterized by the presence of a plasma-cell dyscrasia as a source of monoclonal immunoglobulin light chains, which are toxic and are deposited in multiple organs. Over half of patients (51%-63%) affected by this condition show cardiac involvement at diagnosis,1–3 with the latter being the most important prognostic factor in the natural progression of this disease.4,5
The detection of cardiac involvement has classically relied on either typical findings at endomyocardial biopsy within an appropriate clinical or laboratory context, and/or echocardiographic evidence of amyloidosis associated with a positive result of noncardiac biopsy.6,7 Recent studies have indicated that specific cardiac biomarkers, such as brain natriuretic peptide (BNP) and cardiac troponin, are powerful diagnostic and prognostic tools in AL amyloidosis.1,8 N-terminal pro-B-type natriuretic peptide (NT-proBNP) has been shown to be a sensitive marker of cardiac involvement in amyloid patients, providing incremental value beyond classical electrocardiographic and echocardiographic signs in the discrimination of heart involvement.1 Moreover, this biomarker is an independent predictor of survival in AL amyloidosis1,8 and has been used to classify patients into different prognostic stage groups.8
Together with conventional echocardiography, ultrasound techniques such as tissue Doppler imaging, and more recently, speckle-tracking echocardiography (STE)-derived myocardial mechanics9,10 have demonstrated a potential role in the detection of cardiac involvement and prediction of prognosis in patients with amyloidosis.11–13 While 2-dimensional STE algorithms have been applied to the assessment of cardiac involvement in patients with amyloidosis,13–15 2-dimensional imaging has potential technical limitations due to the inability to track out of plane motion.16 In addition, right ventricular (RV) structure and function, another potentially important prognostic feature,17,18 has only been studied to a very limited extent using this technique. The aim of this study was to characterize 3-dimensional-STE-derived left ventricular (LV) and RV myocardial mechanics in patients with AL amyloidosis and examine the relationship between ventricular mechanics and circulating BNP levels in these patients.
METHODSData were prospectively collected (May 2011 through August 2012) from patients who were undergoing evaluation for amyloidosis at Tufts Medical Center. The study was approved by the institutional review board and study participants provided informed consent. At the time of their evaluation, most of the study participants had been previously diagnosed with AL amyloidosis; in those without a definitive diagnosis at the clinic visit, appropriate tests were performed to either rule out or rule in the disease.
For all participants, the diagnosis of AL amyloidosis was confirmed through positive amyloid staining by Congo red (either fat aspirate, bone marrow or organ biopsy), evidence of light chain-related amyloid (by immunohistochemical staining or immunofluorescence microscopy), and detection of a monoclonal plasma cell proliferative disorder (by serum/urine immunofixation, serum-free light chain ratio analysis, or bone marrow specimen examination). In the aforementioned context, cardiac involvement was considered when at least 1 the following criteria were met: a) positive amyloid staining by Congo red in an endomyocardial biopsy; b) LV wall thickness > 12mm (in the absence of potential causes for the magnitude of the wall thickness increase evident); c) RV free wall thickness > 5mm (in the absence of pulmonary hypertension); d) symptoms of heart failure (graded according to New York Heart Association functional class ≥ II), and e) BNP ≥ 88 ng/L and/or troponin I ≥ 0.1μg/L, consistent with cardiac AL amyloidosis stage II or III involvement by biomarkers.8
EchocardiographyThose patients with either confirmed or suspected (later confirmed) AL amyloidosis underwent a transthoracic echocardiogram on the day of the outpatient clinical evaluation. The study was performed with the commercially available scanner Artida 4D System (Toshiba Medical Systems; Tustin, California, United States). Standard 2-dimensional and Doppler echocardiographic studies were performed with the PST-30SBT transducer, according to the recommendations of the American Society of Echocardiography.19 Subsequently, all study participants underwent 3-dimensional-STE, a technique that has been validated for rendering LV global and regional myocardial mechanics.20
Acquisition of 3-dimensional data sets and off-line analysis for speckle tracking were performed as previously described.21,22 In summary, 3-dimensional data sets consisted of LV full pyramidal volumes, acquired with the Matrix Array PST-25SX transducer from the apical position, and created by the combination of 6 electrocardiography-gated subvolumes. The off-line speckle tracking analysis was performed using the Wall Motion Tracking software (Toshiba Medical Systems) and with the investigator blinded to clinical data. The analysis started with axis adjustment to expose the actual endocardial border; subsequently, semiautomated tracing of endocardial and epicardial borders was rendered by manually marking 6 landmarks on the endocardial border. Then, the automated tracking of borders throughout the cardiac cycle was started, and the 3-dimensional images of the LV walls were automatically divided into a 16-segment model. Finally, the resulting tracings were manually modified only in those areas where the true endocardial and epicardial borders were not correctly tracked. Right ventricle analysis was similar to that of the left ventricle, using placement of multiple reference points (instead of the prefixed landmarks) all over the endocardial boundary to obtain the 3-dimensional tracing of the region of interest (RV myocardium). After automatic tracking of borders, the 3-dimensional images of the RV walls were automatically divided into a 16 segment-model, including 6 basal, 6 midventricular and 4 apical segments. Tracking of endocardial and epicardial tracings was manually modified by the operator if needed.
The quality of tracking was visually judged for each LV segment. Decisions about exclusion of echocardiographic studies for results relied on the discretion of the investigator, and were based both on general 3-dimensional image quality (before attempting analysis) and on accuracy to track the actual LV myocardial motion (during the attempt at analysis). If it was not feasible to either automatically or manually track one or more segments, the case was excluded; thus, all 16 segments from included cases were considered for results.
Myocardial Mechanics ParametersFor the LV analysis, the study focused on peak systolic longitudinal and circumferential strains (LSt and CSt), which represent myocardial deformation in the tangential and circumferential directions, respectively, relative to the endocardial border, having both shown significant reproducibility.20 For each individual, global strain parameters were computed by averaging the peak values corresponding to each of the 16 LV segments. Regional strain parameters were computed by averaging the peak values of specific segments according to basal (6 segments), midventricular (6 segments) and apical (4 segments) levels.
For the RV analysis, the study focused on peak systolic LSt and radial displacement, representing myocardial deformation in the tangential direction and displacement toward the center of the RV cavity, respectively, relative to the endocardial border. These variables were chosen because base-to-apex shortening (longitudinal direction) accounts for most of RV systolic emptying, while inward myocardial motion (radial direction) completes the RV systolic performance.23
Biomarker TestingVenous blood was drawn for BNP determination as part of the comprehensive evaluation for amyloidosis on the day of the outpatient clinical evaluation, prior to the echocardiographic study. Chemiluminescent microparticle immunoassay was performed for determination of plasma BNP levels (ARCHITECT BNP assay; Abbott Diagnostics; Lake Forest, Illinois, United States) in the ARCHITECT i2000SR analyzer (Abbott Diagnostics). The lower limit of detection was 0.5 ng/L. The highest intra- and interassay coefficients of variation range from 3% through 6%.
ReproducibilityFor reproducibility purposes, two 3-dimensional-STE experienced readers were involved in the present work. All RV 3-dimensional data sets were reanalyzed by the same investigator (≥ 8 weeks apart) and by a second blinded investigator. Reproducibility data for LV 3-dimensional-STE-derived parameters has previously been reported by our group (pooled data for intraobserver and interobserver variability, respectively: 5% ± 5% and 6% ± 7% for LSt; 6% ± 6% and 8% ± 9% for CSt).21,24
Statistical AnalysisCategorical variables are expressed as frequencies (percentages). Continuous variables were tested for normality (by using Kolmogorov-Smirnov test) and are shown as mean ± standard deviation or median [interquartile range], as appropriate. The chi-squared test and 1-way analysis of variance (for categorical and continuous variables, respectively) were used for comparisons between groups of patients according to BNP tertiles (first tertile, BNP < 86 ng/L; second tertile, BNP from 86 ng/L to 403 ng/L; third tertile, BNP > 403 ng/L). Following analysis of variance, differences between the corresponding pairs of groups were investigated by the post hoc Turkey HSD (honest significant difference) or Games-Howell tests, as appropriate, according to normality and variance homogeneity of the variables within each tertile. Comparisons between patients with and without cardiac involvement were performed using the unpaired Student t test. After normality the test and construction of scatter plots for pairs of continuous variables, the correlation between them was tested by Pearson and Spearman ρ correlation coefficients, as appropriate. For further examination of both LV global and regional strain parameters, simultaneous multivariate logistic regression analysis was performed using the LV global or regional strain variable with highest association on univariate testing, along with BNP criteria (≥ 88 ng/L) and troponin I criteria (≥ 0.1μg/L) for cardiac involvement, to examine their predictive value for the presence of cardiac involvement. Multivariate analyses were not performed for RV strain parameters due to the number of participants with analyzable RV strain data. Intra- and interobserver variability for RV 3-dimensional-STE parameters was calculated as the absolute difference of the corresponding pair of repeated myocardial mechanics measurements, expressed as a percentage of their mean. A P value<.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics 20.0 (IBM Corp.; Armonk, New York, United States).
RESULTSForty-four patients diagnosed with AL amyloidosis underwent standard echocardiography and 3-dimensional-STE. Four patients were excluded from LV strain analysis due to inability to accurately track myocardial motion, and 18 patients were excluded from RV analysis due to inability to accurately track RV myocardial motion. Therefore, 40 and 26 participants, respectively, formed the patient cohorts with acceptable LV and RV standard echocardiographic and 3-dimensional-STE evaluation. The intra- and interobserver variability obtained for RV 3-dimensional-STE–derived myocardial mechanics parameters was as follows: LSt, 5%±4% and 6%±5%; radial displacement, 7%±5% and 8%±7%. The clinical characteristics of this study population are reported in Table 1.
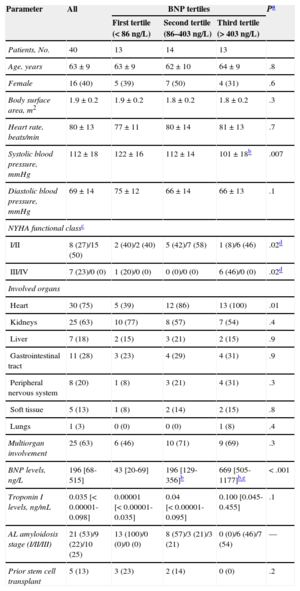
Baseline Clinical Characteristics of Amyloidosis Patients, and According to Brain Natriuretic Peptide Levels
| Parameter | All | BNP tertiles | Pa | ||
|---|---|---|---|---|---|
| First tertile (< 86 ng/L) | Second tertile (86–403 ng/L) | Third tertile (> 403 ng/L) | |||
| Patients, No. | 40 | 13 | 14 | 13 | |
| Age, years | 63±9 | 63±9 | 62±10 | 64±9 | .8 |
| Female | 16 (40) | 5 (39) | 7 (50) | 4 (31) | .6 |
| Body surface area, m2 | 1.9±0.2 | 1.9±0.2 | 1.8±0.2 | 1.8±0.2 | .3 |
| Heart rate, beats/min | 80±13 | 77±11 | 80±14 | 81±13 | .7 |
| Systolic blood pressure, mmHg | 112±18 | 122±16 | 112±14 | 101±18b | .007 |
| Diastolic blood pressure, mmHg | 69±14 | 75±12 | 66±14 | 66±13 | .1 |
| NYHA functional classc | |||||
| I/II | 8 (27)/15 (50) | 2 (40)/2 (40) | 5 (42)/7 (58) | 1 (8)/6 (46) | .02d |
| III/IV | 7 (23)/0 (0) | 1 (20)/0 (0) | 0 (0)/0 (0) | 6 (46)/0 (0) | .02d |
| Involved organs | |||||
| Heart | 30 (75) | 5 (39) | 12 (86) | 13 (100) | .01 |
| Kidneys | 25 (63) | 10 (77) | 8 (57) | 7 (54) | .4 |
| Liver | 7 (18) | 2 (15) | 3 (21) | 2 (15) | .9 |
| Gastrointestinal tract | 11 (28) | 3 (23) | 4 (29) | 4 (31) | .9 |
| Peripheral nervous system | 8 (20) | 1 (8) | 3 (21) | 4 (31) | .3 |
| Soft tissue | 5 (13) | 1 (8) | 2 (14) | 2 (15) | .8 |
| Lungs | 1 (3) | 0 (0) | 0 (0) | 1 (8) | .4 |
| Multiorgan involvement | 25 (63) | 6 (46) | 10 (71) | 9 (69) | .3 |
| BNP levels, ng/L | 196 [68-515] | 43 [20-69] | 196 [129-356]b | 669 [505-1177]b,e | < .001 |
| Troponin I levels, ng/mL | 0.035 [< 0.00001-0.098] | 0.00001 [< 0.00001-0.035] | 0.04 [< 0.00001-0.095] | 0.100 [0.045-0.455] | .1 |
| AL amyloidosis stage (I/II/III) | 21 (53)/9 (22)/10 (25) | 13 (100)/0 (0)/0 (0) | 8 (57)/3 (21)/3 (21) | 0 (0)/6 (46)/7 (54) | — |
| Prior stem cell transplant | 5 (13) | 3 (23) | 2 (14) | 0 (0) | .2 |
AL, systemic amyloidosis in its light chain variant; BNP, brain natriuretic peptide; NYHA, New York Heart Association.
Data are expressed as No. (%), mean±standard deviation or median [interquartile range].
For those with cardiac involvement (total, n=30; first tertile, n=5; second tertile, n=12; third tertile, n=13).
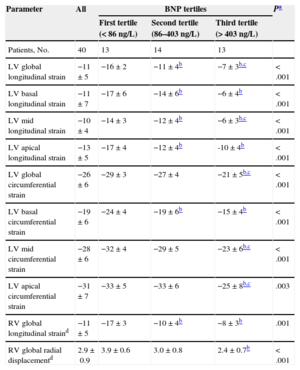
Table 2 shows standard 2-dimensional and Doppler echocardiographic parameters in all study participants, and according to BNP levels. Likewise, Table 3 displays significant differences across BNP level tertiles for all 3-dimensional-STE-derived global and regional myocardial mechanics parameters (Figure 1).
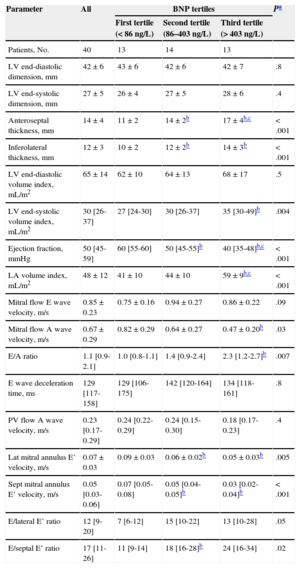
Standard Echocardiographic Parameters in All Amyloidosis Patients, and According to Brain Natriuretic Peptide Levels, at the Time of Clinical Evaluation
| Parameter | All | BNP tertiles | Pa | ||
|---|---|---|---|---|---|
| First tertile (< 86 ng/L) | Second tertile (86–403 ng/L) | Third tertile (> 403 ng/L) | |||
| Patients, No. | 40 | 13 | 14 | 13 | |
| LV end-diastolic dimension, mm | 42±6 | 43±6 | 42±6 | 42±7 | .8 |
| LV end-systolic dimension, mm | 27±5 | 26±4 | 27±5 | 28±6 | .4 |
| Anteroseptal thickness, mm | 14±4 | 11±2 | 14±2b | 17±4b,c | < .001 |
| Inferolateral thickness, mm | 12±3 | 10±2 | 12±2b | 14±3b | < .001 |
| LV end-diastolic volume index, mL/m2 | 65±14 | 62±10 | 64±13 | 68±17 | .5 |
| LV end-systolic volume index, mL/m2 | 30 [26-37] | 27 [24-30] | 30 [26-37] | 35 [30-49]b | .004 |
| Ejection fraction, mmHg | 50 [45-59] | 60 [55-60] | 50 [45-55]b | 40 [35-48]b,c | < .001 |
| LA volume index, mL/m2 | 48±12 | 41±10 | 44±10 | 59±9b,c | < .001 |
| Mitral flow E wave velocity, m/s | 0.85±0.23 | 0.75±0.16 | 0.94±0.27 | 0.86±0.22 | .09 |
| Mitral flow A wave velocity, m/s | 0.67±0.29 | 0.82±0.29 | 0.64±0.27 | 0.47±0.20b | .03 |
| E/A ratio | 1.1 [0.9-2.1] | 1.0 [0.8-1.1] | 1.4 [0.9-2.4] | 2.3 [1.2-2.7]b | .007 |
| E wave deceleration time, ms | 129 [117-158] | 129 [106-175] | 142 [120-164] | 134 [118-161] | .8 |
| PV flow A wave velocity, m/s | 0.23 [0.17-0.29] | 0.24 [0.22-0.29] | 0.24 [0.15-0.30] | 0.18 [0.17-0.23] | .4 |
| Lat mitral annulus E’ velocity, m/s | 0.07±0.03 | 0.09±0.03 | 0.06±0.02b | 0.05±0.03b | .005 |
| Sept mitral annulus E’ velocity, m/s | 0.05 [0.03-0.06] | 0.07 [0.05-0.08] | 0.05 [0.04-0.05]b | 0.03 [0.02-0.04]b | < .001 |
| E/lateral E’ ratio | 12 [9-20] | 7 [6-12] | 15 [10-22] | 13 [10-28] | .05 |
| E/septal E’ ratio | 17 [11-26] | 11 [9-14] | 18 [16-28]b | 24 [16-34] | .02 |
BNP, brain natriuretic peptide; LA, left atrial; LV, left ventricular; PV, pulmonary vein.
Three-dimensional Speckle Tracking Echocardiography-derived Parameters in All Amyloidosis Patients and According to Brain Natriuretic Peptide Levels at the Time of Clinical Evaluation
| Parameter | All | BNP tertiles | Pa | ||
|---|---|---|---|---|---|
| First tertile (< 86 ng/L) | Second tertile (86–403 ng/L) | Third tertile (> 403 ng/L) | |||
| Patients, No. | 40 | 13 | 14 | 13 | |
| LV global longitudinal strain | −11±5 | −16±2 | −11±4b | −7±3b,c | < .001 |
| LV basal longitudinal strain | −11±7 | −17±6 | −14±6b | −6±4b | < .001 |
| LV mid longitudinal strain | −10±4 | −14±3 | −12±4b | −6±3b,c | < .001 |
| LV apical longitudinal strain | −13±5 | −17±4 | −12±4b | -10±4b | < .001 |
| LV global circumferential strain | −26±6 | −29±3 | −27±4 | −21±5b,c | < .001 |
| LV basal circumferential strain | −19±6 | −24±4 | −19±6b | −15±4b | < .001 |
| LV mid circumferential strain | −28±6 | −32±4 | −29±5 | −23±6b,c | < .001 |
| LV apical circumferential strain | −31±7 | −33±5 | −33±6 | −25±8b,c | .003 |
| RV global longitudinal straind | −11±5 | −17±3 | −10±4b | −8±3b | .001 |
| RV global radial displacementd | 2.9±0.9 | 3.9±0.6 | 3.0±0.8 | 2.4±0.7b | < .001 |
BNP, brain natriuretic peptide; LV, left ventricular; RV, right ventricular.
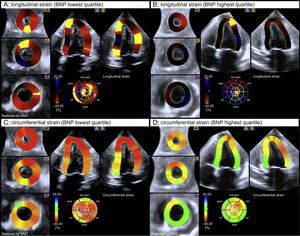
End-systolic left ventricular global longitudinal strain and circumferential strain color-coded 3-dimensional-speckle tracking echocardiography performances in 2 patients with brain natriuretic peptide levels at the lowest (A and C) and the highest (B and D) tertiles. Note the significantly altered myocardial mechanics of the left ventricle in the patient belonging to the highest brain natriuretic peptide level tertile (B and D) according to the color-coded bar. BNP, brain natriuretic peptide. WMT, wall motion tracking.
For the 26 patients with both LV and RV 3-dimensional-STE evaluation, LV and RV global LSt showed excellent correlation (r=0.95; P<.001), while the remaining LV and RV global myocardial mechanics parameters yielded good correlations (LV LSt and RV radial displacement, r=–0.80; P<.001; LV CSt and RV LSt, r=0.78; P<.001; LV CSt and RV radial displacement, r=–0.81; P<.001).
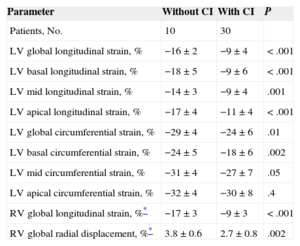
Table 4 displays the results of LV and RV strain analyses in participants with and without cardiac involvement. Left ventricular global LSt and CSt were significantly reduced in patients with cardiac involvement, mainly due to the alteration of basal segments. Thus, the absolute mean difference for basal LSt and CSt between patients with and without cardiac involvement was 9% and 7%, respectively. This represents a relative reduction in basal LSt and CSt due to cardiac involvement of about 50% and 25%, respectively, indicating that the main impairment in deformation associated with cardiac involvement at the basal level occurred in the longitudinal direction. Both RV global LSt and radial displacement were also diminished in patients with cardiac involvement (Figure 2), although LSt showed a significantly greater mean difference between those with and without cardiac involvement (9%). On multivariate analysis, the 3 prespecified candidate variables were LV global LSt (namely, the LV global or regional strain variable with highest association on univariate testing), the presence of cardiac involvement according to BNP levels (≥ 88 ng/L) and the presence of cardiac involvement according to troponin I levels (≥ 0.1μg/L). Left ventricular global LSt was associated with the presence of cardiac involvement independently of the presence of BNP and troponin I criteria for cardiac amyloidosis (odds ratio = 1.6, 95% confidence interval, 1.04-2.37; P=.03).
Three-dimensional Speckle Tracking Echocardiography-derived Parameters in Amyloidosis Patients With and Without Cardiac Involvement
| Parameter | Without CI | With CI | P |
|---|---|---|---|
| Patients, No. | 10 | 30 | |
| LV global longitudinal strain, % | −16±2 | −9±4 | < .001 |
| LV basal longitudinal strain, % | −18±5 | −9±6 | < .001 |
| LV mid longitudinal strain, % | −14±3 | −9±4 | .001 |
| LV apical longitudinal strain, % | −17±4 | −11±4 | < .001 |
| LV global circumferential strain, % | −29±4 | −24±6 | .01 |
| LV basal circumferential strain, % | −24±5 | −18±6 | .002 |
| LV mid circumferential strain, % | −31±4 | −27±7 | .05 |
| LV apical circumferential strain, % | −32±4 | −30±8 | .4 |
| RV global longitudinal strain, %* | −17±3 | −9±3 | < .001 |
| RV global radial displacement, %* | 3.8±0.6 | 2.7±0.8 | .002 |
CI, cardiac involvement; LV, left ventricular; RV, right ventricular.
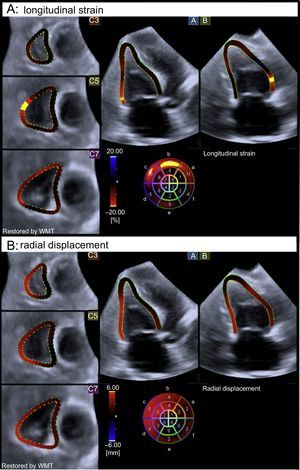
End-systolic right ventricular global longitudinal strain (A) and radial displacement (B) color-coded 3-dimensional-speckle tracking echocardiography performed in a patient with cardiac involvement. Note the significantly altered myocardial mechanics of the right ventricle, according to the color-coded bar. WMT, wall motion tracking.
This is the first study to jointly analyze both LV and RV myocardial mechanics by 3-dimensional-STE; additionally, this study also tested the relationships between myocardial mechanics and circulating BNP levels and cardiac involvement. The current work provides relevant insights into myocardial function in patients with AL amyloidosis as assessed by 3-dimensional-STE: a) LV and RV myocardial mechanics measurements are increasingly altered as BNP levels increase; b) functional impairment of the right and left ventricles appears to follow a parallel behavior based on the linear relationships observed between myocardial mechanics parameters, and c) significantly reduced LSt represents a marker of cardiac involvement beyond circulating BNP and troponin I levels.
Brain natriuretic protein has been found to be a relevant diagnostic and prognostic tool in AL amylodosis.1,8 It likely represents a marker of amyloid-related myocardial toxicity and myocyte functional impairment due to amyloid deposition.25 Those 2 mechanisms of myocardial damage may determine alterations in LV and RV myocardial mechanics11,12 that can be measured by STE.13–15 Thus, as BNP is released from both ventricles due to light-chain toxicity and amyloid deposition, myocardial deformation is proportionally affected, as demonstrated by its association with the biomarker (Table 3). A post hoc analysis of the relationships between troponin I tertiles and myocardial mechanics was also performed (troponin I testing information of the ). The results of this analysis () followed the relationship demonstrated for BNP, with LV and RV myocardial mechanics increasingly altered as troponin I levels increase. However, standard deviations corresponding to the strain and displacement mean values across troponin I tertiles were larger than those observed for BNP. Based on these data, BNP seems to better differentiate between different degrees of myocardial mechanics impairment. In this regard, comparison of BNP levels with relatively novel high sensitivity troponin levels is warranted.3
Circumferential function, mainly derived from shortening of epicardial muscle fibers, has been shown to help in maintaining systolic function in different cardiovascular conditions with the greatest involvement of endocardial muscle fibers.24 In the present work, both longitudinal and circumferential functions were altered when cardiac involvement was present (Table 4), which was likely related to the transmural amyloid deposition throughout the LV myocardium. However, circumferential shortening was less impaired than longitudinal shortening, and so the former might still represent a less sensitive vector of deformation, similar to other conditions, in response to a particular insult (hemodynamic, ischemic, structural, or toxic) on the myocardium.24 It is also noteworthy that the alteration in myocardial mechanics for both longitudinal and circumferential shortenings was most striking at the LV basal segments (Table 4); these findings are consistent with prior reported observations regarding regional LSt in light-chain amyloidosis.26 Although this study was not designed to elucidate aspects about LV global and regional systolic dysfunction in amyloidosis, it serves as a hypothesis-generating work in terms of the diagnostic and even prognostic potential of these findings.
Evaluation of RV myocardial involvement in AL amyloidosis is scarce and challenging compared with echocardiographic assessment of LV wall thickness as a criterion for cardiac involvement.17,18 There is controversy regarding the timing of RV functional impairment, and whereas some have reported RV dysfunction at early stages,27 others have suggested that it evolves later than LV amyloid deposition.28 Nevertheless, there appears to be agreement that RV dysfunction cannot be explained on the basis of amyloid deposition alone, and that the hemodynamic and histologic RV and LV interdependence plays a major role in RV performance.18,28 The present work suggests that RV systolic functional impairment runs parallel to that of the left ventricle, both in its longitudinal shortening (RV LSt) and in its inward myocardial motion (RV radial displacement). Regardless of the underlying mechanism, examination for RV dysfunction should be part of the echocardiographic evaluation in AL amyloidosis.17,18 The assessment of a geometrically complex structure such as the right ventricle through 3-dimensional-STE-derived myocardial mechanics may provide new insights into the timing and extent of RV functional impairment in AL amyloidosis.
Despite the existing literature on the diagnostic and prognostic role of natriuretic peptides and troponins in AL amyloidosis, the dynamic nature of circulating biomarkers should be taken into consideration when assessing cardiac involvement. Thus, an increased level of natriuretic peptides or troponins can be used as a marker of cardiac involvement at some point of the disease course, but following treatment or patient stabilization, circulating levels of the same biomarker may return to normal values or lower values. This might explain why LV LSt impairment was associated with cardiac involvement independent of BNP and troponin I levels in our cohort of patients, given their different disease stages at the time of evaluation. The reduction in the prognostic value of circulating biomarker levels following treatment strategies in AL amyloidosis has been suggested elsewehere.29 Therefore, the hypothesis of a greater diagnostic and prognostic power of longitudinal shortening assessment throughout the disease course, and not only before treatment is started, is a topic that warrants investigation.
LimitationsOne limitation of this study is its small sample size, particularly in relation to RV myocardial mechanics. In addition, the statistical power of the LV-related logistic regression analysis might be low to detect other independent associations with cardiac involvement, that is, BNP and/or troponin I levels; these biomarkers should still be regarded as potential tools for the detection of cardiac involvement. Nevertheless, LV analysis through 3-dimensional-STE yielded outcomes that are consistent with previous observations and in keeping with general knowledge from the existing literature. Although the feasibility of LV 3-dimensional-STE analysis was 90% (40 of 44), which is quite high and likely due to the good tissue-blood ultrasound interface at endocardial level in amyloidosis cases, RV 3-dimensional-STE analysis only reached 59% (26 of 44); a likely source of this limitation was the use of a nondedicated software for RV 3-dimensional-STE analysis and the anterior position of the right ventricle behind the sternum, which may impose technical limitations on imaging.
CONCLUSIONSThe current work provides relevant insights into myocardial function in patients with AL amyloidosis as assessed by 3-dimensional-STE. Three-dimensional STE-derived LV and RV myocardial mechanics, which appear to follow a parallel behavior, are increasingly altered as BNP levels increase. Among LV myocardial mechanics measurements, global LSt appears to have a strong association with cardiac involvement, beyond circulating biomarkers such as BNP and troponin I.
FUNDINGJ.A. Urbano-Moral has received a research grant from Fundación Alfonso Martin Escudero (Madrid, Spain).
CONFLICTS OF INTERESTN.G. Pandian has received lecture fees from Toshiba Medical Systems. The Cardiovascular Imaging and Hemodynamic Laboratory at Tufts Medical Center has received an equipment grant from Toshiba Medical Systems (Tustin, California, United States).