Mechanical response to myocardial stretch has been explained by various mechanisms, which include Na+/H+ exchanger activation by autocrine-paracrine system activity. Drug-induced changes were analyzed to investigate the role of these mechanisms in the electrophysiological responses to acute myocardial stretch.
MethodsMultiple epicardial electrodes and mapping techniques were used to analyze changes in ventricular fibrillation induced by acute myocardial stretch in isolated perfused rabbit hearts. Four series were studied: control (n = 9); during perfusion with the angiotensin receptor blocker losartan (1 μM, n = 8); during perfusion with the endothelin A receptor blocker BQ-123 (0.1 μM, n = 9), and during perfusion with the Na+/H+ exchanger inhibitor EIPA (5-[N-ethyl-N-isopropyl]-amiloride) (1 μM, n = 9).
ResultsEIPA attenuated the increase in the dominant frequency of stretch-induced fibrillation (control=40.4%; losartan=36% [not significant]; BQ-123=46% [not significant]; and EIPA=22% [P<.001]). During stretch, the activation maps were less complex (P<.0001) and the spectral concentration of the arrhythmia was greater (greater regularity) in the EIPA series: control=18 (3%); EIPA = 26 (9%) (P < .02); losartan=18 (5%) (not significant); and BQ-123=18 (4%) (not significant).
ConclusionsThe Na+/H+ exchanger inhibitor EIPA attenuated the electrophysiological effects responsible for the acceleration and increased complexity of ventricular fibrillation induced by acute myocardial stretch. The angiotensin II receptor antagonist losartan and the endothelin A receptor blocker BQ-123 did not modify these effects.
Keywords
The mechanical response of myocytes to stretch has been explained by various mechanisms, which include the local release of angiotensin II and endothelin, Na+/H+ exchanger activation, increased Na+ influx, Na+/Ca2+ exchanger reverse mode activation, and increased Ca2+ transients.1,2 There is little information on the role of these mechanisms in the electrophysiological responses to myocardial stretch (electromechanical feedback) or on pharmacological modifications of the proarrhythmic effects of stretch.3–10
Inhibition of the Na+/H+ or Na+/Ca2+ exchangers decreases the slow inotropic response to stretch and the magnitude of Ca2+ transients.1,2,11–14 In turn, in relation to electromechanical feedback, the Na+/Ca2+ exchanger inhibitor, KB-R7943, reduces the electrophysiological changes induced by stretch.5 However, it remains unknown whether these changes are also reduced by the inhibition of the Na+/H+ exchanger or by blocking the effects of substances that may be involved in its activation after myocardial stretch, such as angiotensin II or endothelin.1,15
An experimental model was used to obtain more information on the mechanisms involved in the electrophysiological responses to myocardial stretch and its pharmacological modifications. The characteristics of myocardial activation during ventricular fibrillation (VF) can be analyzed to determine the time course of changes in electrophysiological myocardial properties caused by acute myocardial stretch applied in the left ventricular free wall.5,16,17 The objectives of this study were: a) to determine whether the inhibition of the Na+/H+ exchanger, whose activation during stretch is a prior step to Na+/Ca2+ reverse mode activation, also blocks or attenuates electrophysiological responses to stretch, and b) to determine whether the inhibition of angiotensin II type I receptors or endothelin A receptors, whose activation is thought to intervene in the mechanical response of myocytes to stretch, also modifies the manifestations of electromechanical feedback in the experimental model.
METHODSExperimental PreparationThis study fulfilled the recommendations of the European Union directive 2010/63/EU on animal experimentation. New Zealand rabbits were premedicated with ketamine, administered heparin, and killed with sodium thiopental. After the heart was removed, the aorta was cannulated using a Langendorff system to perfuse oxygenated Tyrode at 80mmHg and 37 (0.5) oC.
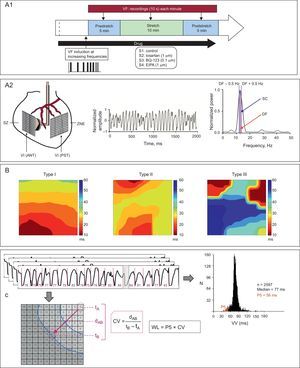
As described in previous studies,5,16–18 a device was placed in the left ventricular cavity via the atrium to induce stretch in a specific area of the ventricular wall. Two multiple electrodes comprising 121 and 115 stainless steel unipolar electrodes (interelectrode distance=1mm) were positioned in the epicardium of the anterior wall (stretch zone) and the rear wall (nonstretch zone) (Figure 1). Recordings and stimulation techniques were similar to those described in the cited studies.
A.1: outline of the experimental protocol. A.2: location of multiple electrodes and device used for myocardial stretch, and examples of recording and spectral analysis. B: types of activation maps according to their complexity. C: electrophysiological parameters used. CV, conduction velocity; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; DF, dominant frequency; dAB, distance between 2 electrodes in the direction of the activation front (perpendicular to the isochrones); LV (ANT), left ventricular anterior wall; LV (PST), left ventricular posterolateral wall; NSZ, nonstretch zone; P5, 5th percentile; SC, spectral concentration; SZ, stretch zone; tA and tB, activation times in the electrodes A and B; VF, ventricular fibrillation; WL, wavelength; VV, median of the consecutive activation intervals during ventricular fibrillation.
Four series were studied: a) control (n=9); b) during perfusion with the angiotensin receptor blocker losartan (1μM, n=8); c) during perfusion with the endothelin A receptor blocker BQ-123 (0.1μM, n=9); and d) during perfusion with the Na+/H+ exchanger inhibitor EIPA (5-[N-ethyl-N-isopropyl]-amiloride) (1μM, n=9). The concentration of these substances was within the ranges used in experimental studies,15,19-22 and perfusion was started 15 min before electrophysiological study.
In each series, 30min after placing the electrodes, VF was induced by stimulation at increasing frequencies while coronary perfusion was maintained. Five minutes after VF was induced, stretch was applied at longitudinal increments of 12% in the vertical and horizontal axes of the modified zone.16 Local stretch was suppressed after 10 min.
Data AnalysisVentricular Fibrillation Spectral AnalysisThe Welch method23 was used to obtain the power spectrum of the signals recorded with each unipolar electrode located in the 2 study sites. The spectral analysis was performed each minute before stretch induction, during stretch, and after stretch suppression (Figure 1). The spectrum corresponded to the first 4s of each record (4096 points, sampling rate=1kHz) The dominant frequency (DF) in each electrode was obtained by determining the maximum value of the power spectral density. In addition, spectral concentration (SC) was calculated as the percentage of total energy within the DF range (0.5Hz).
Time-domain AnalysisThe methodology described above5,16–18 was used to determine local activation times in each electrode. The median of the consecutive activation intervals during VF (VV) and the 5th percentile (P5) in each electrode were determined during 2-s time windows at baseline, 3min after stretch induction, and 3min after stretch suppression. These 3 time windows were chosen after performing the spectral analysis, thus enabling the rapid determination of the moment of maximum effect during stretch and the time interval until these effects disappeared.
Activation MapsAs described in previous studies,5,16,17,24 activation maps during VF were constructed every 100ms in the 3 time windows analyzed. Each map was classified according to its complexity: low (type I), intermediate (type II), or high (type III) (Figures 1 and 2). A breakthrough pattern was defined as the earliest activation in the multielectrode area with centrifugal propagation. The conduction velocity during VF was determined by dividing the distance between 2 electrodes separated by 5 interelectrode spaces in a direction perpendicular to the isochrones by the difference between their activation times (average of 3 calculations) (Figure 1). The functional refractory period during VF was determined by calculating the P5 of the VV intervals, where P5 is an estimation of the shortest intervals.16 The wavelength of the activation process during VF was calculated as the product of conduction velocity and P5.
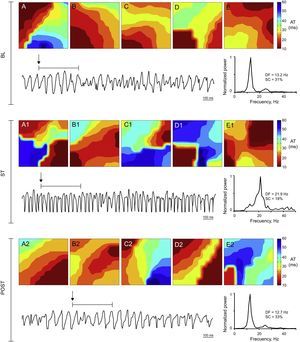
Activation maps, ventricular fibrillation recording obtained with 1 of the electrodes, and power spectrum of the signal recorded in the stretch zone immediately before stretch, during stretch, and poststretch in a control experiment. During stretch ventricular fibrillation accelerates, the dominant frequency increases, and more complex activation maps (type III) predominate. AT, activation time; BL, baseline; DF, dominant frequency; POST, poststretch; SC, spectral concentration; ST, stretch.
Continuous variables are presented as means (standard deviation) and discrete variables are presented as percentages. The general linear model was used to analyze differences in each series (within-subjects differences, repeated measures: baseline, stretch, poststretch) and to compare the series (between-subjects differences). The chi-square test was used to analyze differences between qualitative variables. A P-value of<.05 was used as a cutoff for statistical significance. The data were analyzed using the SPSS 19.0 software package.
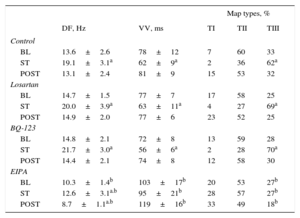
RESULTSEffects of Stretch on Activation Frequency During Ventricular FibrillationThe Table shows the results of each series. In all series, the DF significantly increased during stretch. However, the magnitude of increase was lower in the EIPA series (Figure 3). The percentage increase from baseline was 40.4% in the control series, 36% in the losartan series (not significant [ns] vs control), 46% in the BQ-123 series (ns vs control), and 22% in the EIPA series (P<.001 vs control). The baseline DF was similar in the control, losartan, and BQ-123 series, but lower in the EIPA series (P<.01 vs control). No differences were found between the control, losartan, and BQ-123 series in the maximum DF reached during stretch, whereas the maximum DF reached was lower in the EIPA series (P<.0001 vs control). Similarly, after stretch suppression, significant differences were found between the control and EIPA series (P<.0001).
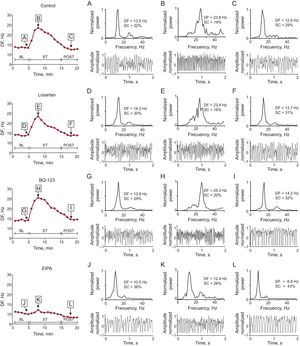
Dominant frequency during ventricular fibrillation recorded with 1 of the electrodes located in the stretch zone in 1 experiment in each series. The power spectrum of the signal recorded before stretch, 3minutes after stretch induction, and 3minutes after stretch suppression. For reasons of clarity, 2-second recordings of ventricular fibrillation are shown, although the spectrogram was obtained from data blocks of 4096 points at a 1kHz sampling rate. BL, baseline; DF, dominant frequency; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; POST, poststretch; SC, spectral concentration; ST, stretch; VF, ventricular fibrillation.
The Table also shows the results of calculating VV. A significant decrease in VV during stretch was observed in the control, losartan, and BQ-123 series. This decrease did not reach statistical significance in the EIPA series. At baseline, the VV value was similar in the control, losartan, and BQ-123 series but higher in the EIPA series (P<.002 vs control). During stretch, the decrease in VV was similar in the control, losartan, and BQ-123 series but smaller in the EIPA series (P<.0001 vs control). After stretch suppression, VV values were higher in the EIPA series than in the control series (P<.0001).
During and after stretch, no significant differences were found in the nonstretch zone in baseline DF and VV values except in the EIPA series, in which DF was lower and VV was higher after stretch suppression, reaching similar values to those observed in the stretch zone.
Effects of Stretch on the Organization of Ventricular FibrillationSpectral ConcentrationDuring stretch, SC values decreased in the stretch zone in the 4 series (Figures 2–4). At baseline, no differences were observed between the control and experimental series. During stretch, higher SC values were observed in the EIPA series (P<.02) than in the control series, whereas the decrease observed in SC values was similar in the losartan, BQ-123, and control series. After stretch, SC was higher in the EIPA series (P<.02) than in the control series. During and after stretch, no significant differences in baseline values were observed in the nonstretch zone, except in the EIPA series, in which a significant increase was observed in the poststretch phase.
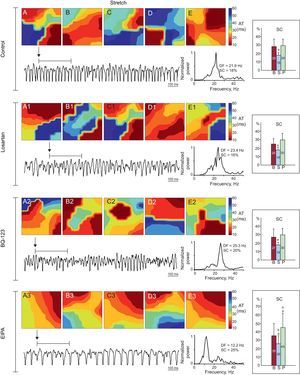
Activation maps obtained during stretch in 1 experiment in each series. There is a predominance of complex activation maps (type III) in the control, losartan, and BQ-123 series, whereas simpler maps were obtained in the EIPA series. The dominant frequency is lower in EIPA series recordings. The right-hand side of the figure shows the averages of spectral concentration in each of the 3 phases of the series of experiments. AT, activation time; B, baseline; DF, dominant frequency; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; P, poststretch; S, stretch; SC, spectral concentration. aSignificantly different compared to baseline. bSignificantly different compared to the control series.
In the control, losartan, and BQ-123 series, stretch similarly increased the complexity of ventricular activation during VF (P<.0001), which was assessed by the percentage of map types; there was an increase in type III maps and a decrease in type I and II maps (Table). Complexity did not increase during the stretch in the EIPA series. Before stretch, no significant differences were found in the control, losartan, and BQ-123 series, whereas complexity was less in the EIPA series (P<.02). During stretch, VF activation was less complex in the EIPA series (P<.0001) than in the control series (Figure 4).
Dominant Frequency, Consecutive Activation Intervals During Ventricular Fibrillation, and Types of Activation Maps During Ventricular Fibrillation Obtained in Each Experimental Series Before, During, and After Stretch
| Map types, % | |||||
|---|---|---|---|---|---|
| DF, Hz | VV, ms | TI | TII | TIII | |
| Control | |||||
| BL | 13.6±2.6 | 78±12 | 7 | 60 | 33 |
| ST | 19.1±3.1a | 62±9a | 2 | 36 | 62a |
| POST | 13.1±2.4 | 81±9 | 15 | 53 | 32 |
| Losartan | |||||
| BL | 14.7±1.5 | 77±7 | 17 | 58 | 25 |
| ST | 20.0±3.9a | 63±11a | 4 | 27 | 69a |
| POST | 14.9±2.0 | 77±6 | 23 | 52 | 25 |
| BQ-123 | |||||
| BL | 14.8±2.1 | 72±8 | 13 | 59 | 28 |
| ST | 21.7±3.0a | 56±6a | 2 | 28 | 70a |
| POST | 14.4±2.1 | 74±8 | 12 | 58 | 30 |
| EIPA | |||||
| BL | 10.3±1.4b | 103±17b | 20 | 53 | 27b |
| ST | 12.6±3.1a.b | 95±21b | 28 | 57 | 27b |
| POST | 8.7±1.1a.b | 119±16b | 33 | 49 | 18b |
BL, baseline, before stretch; DF, dominant frequency of ventricular fibrillation; POST, poststretch; ST, during stretch; TI, TII, TIII: type I, II, and III maps; VV, median of the consecutive activation intervals during ventricular fibrillation.
During stretch, no significant difference was found in the percentage of maps with breakthrough patterns compared to baseline values in the control series (baseline 23%, during stretch 32%, poststretch 26%, ns), the losartan series (baseline 24%, during stretch 20%, poststretch 31%, ns), the BQ-123 series (baseline 27%, during stretch 21%, poststretch 18%, ns) and the EIPA series (baseline 31%, during stretch 22%, poststretch 21%, ns). In all 3 phases, the percentages were similar to those obtained in the control series.
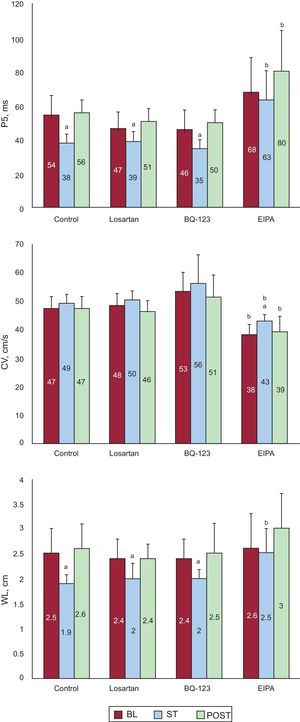
Effect of Stretch on Electrophysiological Parameters During Ventricular FibrillationFigure 5 shows the P5 values obtained in the 4 series. During stretch, the P5 values significantly decreased in all series except in the EIPA series. At baseline, the P5 values were similar in the control, losartan, and BQ-123 series, whereas the P5 value was slightly higher in the EIPA series, but without reaching statistical significance (P<.07). During stretch, the P5 values similarly decreased in the control, losartan, and BQ-123 series, whereas the P5 value was higher in the EIPA series (P<.0001) than in the control series. No statistically significant differences were found in conduction velocities at baseline and during stretch except in the EIPA series (Figure 5). No statistically significant differences were found in the control series at baseline, during stretch, and after stretch except in the EIPA series. During stretch, the wavelength of the VF activation process significantly decreased in the control, losartan, and BQ-123 series, but not in the EIPA series, whereas the wavelength remained significantly longer in the EIPA series than in the control series.
Mean values of the 5th percentile of the consecutive activation intervals during ventricular fibrillation, conduction speed, and the wavelength of the activation process obtained in the stretch zone in the 3 experimental phases of the 4 series. BL, baseline; CV, conduction velocity; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; P5, 5th percentile; POST, poststretch; ST, stretch; WL, wavelength. aSignificantly different compared to baseline. bSignificantly different compared to the control series.
The main results of the study are: a) EIPA attenuates the electrophysiological effects induced by acute myocardial stretch; and b) losartan and BQ-123 do not modify these effects.
Effects of EIPAMyocardial stretch increases Na+ influx into the myocyte. Different mechanisms can be involved,11,14,15,25–28 including the activity of stretch-activated channels,11,25–28 the triggering of autocrine-paracrine mechanisms, which activates angiotensin II and endothelin receptors and activates the Na+/H+ exchanger,2,15 and the increase in Na+/H+ activity mediated by mechanical stimulation.14 In the present study, the Na+/H+ exchanger inhibitor EIPA attenuated the electrophysiological effects induced by acute myocardial stretch, similar to the way in which the inhibition of this exchanger attenuates the slow increase in the contractile force after stretch.2,11,13,14 The effect of EIPA on the Na+/H+ exchanger and on the increase in Na+ intracellulular concentration decreases Na+/Ca2+ exchanger reverse mode activation and its effects on electromechanical feedback. The attenuation of these effects by the Na+/Ca2+ exchanger inhibitor KB-R7943 has been described in previous work.5 The decrease in Na+/Ca2+ exchanger reverse mode activity would reduce Ca2+ influx during stretch and thereby the changes in cellular electrophysiological properties28–30 and the characteristics of the electrical restitution curve that relates the action potential duration with the preceding diastolic interval.31
However, EIPA is not only an Na+/H+ exchanger inhibitor,32,33 and its action on other ionic currents could also be involved in the effects observed. The frequency-dependent block of the fast sodium current under the action of EIPA has been described in previous work.32 Although at a concentration of 1μM a slight reduction in the current was observed in this study, the frequency of rapid activation during VF could increase this effect. On the other hand, EIPA, in the same manner as amiloride, may reduce the persistent sodium current and thus act on the increase in Na+ influx induced by stretch. In the present study, there was a decrease in arrhythmia and in conduction velocity in the EIPA series. There was also a decrease in VF complexity before stretch. This effect was more pronounced in the poststretch phase and was probably due to a cumulative effect on refractoriness during the drug-perfusion period.
During stretch in the EIPA series, no increase was observed in the complexity of the maps and there were no significant changes in P5 and wavelength. There was a 26% reduction in SC compared to baseline, although this reduction was lower than that observed in the control series (36%). However, during stretch, SC values were significantly higher than in the control series and similar to those obtained in the control series before stretch. These results suggest that EIPA attenuates the reduction in the regularity and homogeneity of electrograms induced by stretch. The reduced heterogeneity of the arrhythmia during stretch in the EIPA series was more evident when there were no significant changes in the complexity of the activation maps. This behavior was similar to that observed in other parameters such as P5 and wavelength. Thus, SC was the parameter most sensitive to stretch, indicating that the regularity and homogeneity of the electrograms were also affected by stretch in the EIPA series, but less than in the control series. If the EIPA-induced modifications of the effects of stretch are taken into account, the greater homogeneity of activation during VF could indicate a more favorable treatment outcome regarding actions aimed at interrupting VF, but it would also be more difficult to induce VF in the presence of arrhythmogenic factors such as myocardial stretch, although the analysis of these effects is beyond the scope of this study.
Effects of Losartan and BQ-123The slow increase in the contractile force in response to myocardial stretch has been linked to autocrine-paracrine system activation, following the observation that it is abolished by blocking the angiotensin II type 1 and endothelin A receptors.2,15 It has been suggested that the release of angiotensin II induced by myocardial stretch activates the release of endothelin which, through intervening mechanisms, leads to Na+/H+ exchanger activation, thereby leading to an increase in Na+ influx and the subsequent activation of the Na+/Ca2+ exchanger reverse mode.1,2,15 The increase in Ca2+ transients would be responsible for inotropic response to mechanical stretch. Another study also found that the slow inotropic response to acute stretch in rabbit papillary muscles was mediated by the activation of angiotensin type 1 receptors and by its effectors the Na+/H+ and Na+/Ca2+ exchangers in reverse mode.34 However, some authors have found that the slow inotropic response is not abolished by blocking the angiotensin II receptors12,14,35 or modified by blocking the endothelin receptors.28 A possible explanation for these discrepancies would be differences between species or experimental designs,2,14 although different mechanisms could result in similar outcomes.2 The present study investigated whether there were similarities in the mechanisms involved in mechanical and electrophysiological responses after acute myocardial stretch. It was found that blocking angiotensin II type 1 receptors with losartan and endothelin A receptors with BQ-123 did not modify VF acceleration or the increase in the complexity of the arrhythmia produced by myocardial stretch. The activation of angiotensin II and endothelin receptors does not appear to be involved in the chain of events leading to the electrophysiological manifestations of the electromechanical feedback observed in the experimental model.
LimitationsAny results depend on the characteristics and conditions of the experimental preparations to which acute myocardial stretch is applied. The effects of stretch in chronic preparations and in situ heart preparations can lead to different manifestations, which are caused by associated neurohumoral reflexes, among other factors. Potential interspecies differences should also be taken into account when extrapolating the results obtained.
CONCLUSIONSIn the experimental model, the Na+/H+ exchanger inhibitor EIPA attenuated the electrophysiological effects responsible for the acceleration and the increased complexity of VF induced by acute myocardial stretch. The angiotensin II receptor antagonist losartan and the endothelin A receptor blocker BQ-123 did not modify these effects.
FUNDINGThis study was funded by the Spanish Department of Science (Instituto de Salud Carlos III): projects FIS PS09/02417, FIS PI12/00407, and RETIC “RIC” RD12/0042/0048, and Generalitat Valen-ciana: project PROMETEO 2010/093.
CONFLICTS OF INTERESTNone declared.