The presence of severe neurological sequels following sudden cardiac arrest leads to heavy resource use. Therapeutic hypothermia is indicated to prevent severe brain damage (SBD)1 although this treatment2,3 and prognostic evaluation in this setting are controversial. Prognostic evaluation basically has 2 objectives: first, to inform the family of the possibilities of recovery and, second, to aid diagnostic and therapeutic decisions. A neurological evaluation using several variables is recommended from the third day after injury. In contrast, information on the early prediction of SBD has received much less attention.
The aim of this study was to design a model for predicting SBD using the admission data of consecutive patients who survived cardiac arrest of probable cardiac origin and who underwent therapeutic hypothermia.
Data from patients admitted to the cardiology intensive care unit at our center were collected between November 2009 and January 2014. Their clinical and analytical data were recorded, as well as their clinical course while in hospital. Hypothermia was applied using an Artic-Sun® device (33° C 24-hour and rewarming at 0.25°C/h). Neurological outcome was measured using the Cerebral Performance Category (CPC) scale. Patients with a CPC 3 to 5 and those who died during hypothermia were considered to have SBD. In patients with no recovery of consciousness after completing hypothermia and withdrawal of sedation, an electroencephalogram was performed 72 to 96 hours after admission, as well as an evoked potentials test to determine the presence of the N20 wave, which indicates cortical response.
A model for predicting SBD was designed using various variables available early on. The analysis included the variables available at admission that showed a statistical association (P < .2) with the onset of SBD. The predictive model was obtained using binary logistic regression and ROC curve analysis (PASW Statistics 19.0; Chicago, Illinois, United States), prioritizing the simplicity of the measure and the reproducibility of its component variables, as well as the statistical criteria of the lowest Mallow's Cp, the greatest area under the ROC curve (AUC), and maximum parsimony of the model.
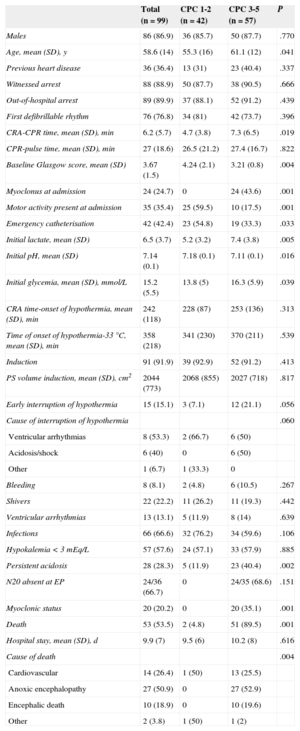
Of the total number of patients treated (n = 100), 1 was excluded for being deemed unsuitable. The patient characteristics and their in-hospital clinical course are summarized in the Table.
Clinical Characteristics, Handling and Clinical Outcome Based on Neurological Course
| Total (n = 99) | CPC 1-2 (n = 42) | CPC 3-5 (n = 57) | P | |
|---|---|---|---|---|
| Males | 86 (86.9) | 36 (85.7) | 50 (87.7) | .770 |
| Age, mean (SD), y | 58.6 (14) | 55.3 (16) | 61.1 (12) | .041 |
| Previous heart disease | 36 (36.4) | 13 (31) | 23 (40.4) | .337 |
| Witnessed arrest | 88 (88.9) | 50 (87.7) | 38 (90.5) | .666 |
| Out-of-hospital arrest | 89 (89.9) | 37 (88.1) | 52 (91.2) | .439 |
| First defibrillable rhythm | 76 (76.8) | 34 (81) | 42 (73.7) | .396 |
| CRA-CPR time, mean (SD), min | 6.2 (5.7) | 4.7 (3.8) | 7.3 (6.5) | .019 |
| CPR-pulse time, mean (SD), min | 27 (18.6) | 26.5 (21.2) | 27.4 (16.7) | .822 |
| Baseline Glasgow score, mean (SD) | 3.67 (1.5) | 4.24 (2.1) | 3.21 (0.8) | .004 |
| Myoclonus at admission | 24 (24.7) | 0 | 24 (43.6) | .001 |
| Motor activity present at admission | 35 (35.4) | 25 (59.5) | 10 (17.5) | .001 |
| Emergency catheterisation | 42 (42.4) | 23 (54.8) | 19 (33.3) | .033 |
| Initial lactate, mean (SD) | 6.5 (3.7) | 5.2 (3.2) | 7.4 (3.8) | .005 |
| Initial pH, mean (SD) | 7.14 (0.1) | 7.18 (0.1) | 7.11 (0.1) | .016 |
| Initial glycemia, mean (SD), mmol/L | 15.2 (5.5) | 13.8 (5) | 16.3 (5.9) | .039 |
| CRA time-onset of hypothermia, mean (SD), min | 242 (118) | 228 (87) | 253 (136) | .313 |
| Time of onset of hypothermia-33°C, mean (SD), min | 358 (218) | 341 (230) | 370 (211) | .539 |
| Induction | 91 (91.9) | 39 (92.9) | 52 (91.2) | .413 |
| PS volume induction, mean (SD), cm2 | 2044 (773) | 2068 (855) | 2027 (718) | .817 |
| Early interruption of hypothermia | 15 (15.1) | 3 (7.1) | 12 (21.1) | .056 |
| Cause of interruption of hypothermia | .060 | |||
| Ventricular arrhythmias | 8 (53.3) | 2 (66.7) | 6 (50) | |
| Acidosis/shock | 6 (40) | 0 | 6 (50) | |
| Other | 1 (6.7) | 1 (33.3) | 0 | |
| Bleeding | 8 (8.1) | 2 (4.8) | 6 (10.5) | .267 |
| Shivers | 22 (22.2) | 11 (26.2) | 11 (19.3) | .442 |
| Ventricular arrhythmias | 13 (13.1) | 5 (11.9) | 8 (14) | .639 |
| Infections | 66 (66.6) | 32 (76.2) | 34 (59.6) | .106 |
| Hypokalemia < 3 mEq/L | 57 (57.6) | 24 (57.1) | 33 (57.9) | .885 |
| Persistent acidosis | 28 (28.3) | 5 (11.9) | 23 (40.4) | .002 |
| N20 absent at EP | 24/36 (66.7) | 0 | 24/35 (68.6) | .151 |
| Myoclonic status | 20 (20.2) | 0 | 20 (35.1) | .001 |
| Death | 53 (53.5) | 2 (4.8) | 51 (89.5) | .001 |
| Hospital stay, mean (SD), d | 9.9 (7) | 9.5 (6) | 10.2 (8) | .616 |
| Cause of death | .004 | |||
| Cardiovascular | 14 (26.4) | 1 (50) | 13 (25.5) | |
| Anoxic encephalopathy | 27 (50.9) | 0 | 27 (52.9) | |
| Encephalic death | 10 (18.9) | 0 | 10 (19.6) | |
| Other | 2 (3.8) | 1 (50) | 1 (2) |
CPC, Cerebral Performance Category scale; CRA, cardiorespiratory arrest; EP, evoked potentials; CPR, cardiopulmonary resuscitation, PS, physiological saline.
Unless otherwise indicated, the data express n (%), n/N (%) (N are the patients who were examined).
Persistent acidosis was defined as that which persisted for 6hours or more despite treatment. Significant bleeding events were defined as those that caused haemodynamic instability or required intervention, transfusion of blood derivatives or suspension of antithrombotic treatment.
The incidence of SBD was 57 of 99 (57.6%). The distribution by CPC categories at discharge was: CPC1, 34 of 99 (34.3%); CPC2, 8 of 99 (8.1%); CPC3, 5 of 99 (5.1%); CPC4, 38 of 99 (38.4%); and CPC5, 12 of 99 (12.1%); the 2 remaining patients (2.1%) died before reaching normothermia.
At admission, the variables associated with SBD were initial lactate level, metabolic acidosis, myoclonus, absence of motor activity, time to start of cardiopulmonary resuscitation, and age. Other variables associated with SBD were myoclonic status at the end of hypothermia, persistent metabolic acidosis, and the absence of cortical response in evoked potentials.
The technical aspects and complications associated with the treatment were not correlated to SBD.
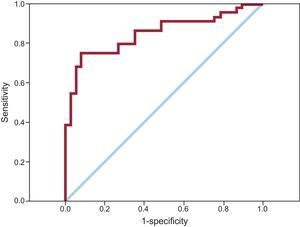
The final predictive model, comprising 3 components (age, initial lactate, and myoclonus at admission) showed an AUC of 0.85 (95% confidence interval, 0.76-0.94). The Figure shows the ROC curve of the predictive model.
In patients undergoing therapeutic hypothermia, neurological recovery may be delayed by the effect of sedation or by therapeutic hypothermia in the brain. Current recommendations stipulate that neurological evaluation should be delayed for more than 72hours and should be based on multiple predictors, as no individual variable can completely rule out a delayed recovery.
There is little information on early prognostic evaluation in patients with sudden cardiac arrest. Recently, Aschauer et al4 analyzed a series of 1932 patients with out-of-hospital sudden cardiac arrest and obtained a risk of death score at 30 days with 4 variables with a remarkable predictive capacity (AUC = 0.81). The main difference with our series was the objective variable. In our opinion, all-cause mortality (bleeding, infections, etc) and SBD are outcomes with significant conceptual differences and different socioeconomic consequences. In addition, some of the variables included, despite being powerful predictors (amount of adrenaline administered, minutes to recovery of spontaneous circulation), are sometimes difficult to measure accurately given the emerging nature of the event. In our series, priority was given to the simplicity, objectivity, and reproducibility of the variable measurement, while recognizing the existence of multiple SBD predictors, as shown in other studies. The model obtained was parsimonious, using only 3 predictors and optimal predictive capacity.
Nevertheless, the practical applicability of these findings involves the design of a risk score by weighing up the importance of each variable and establishing bands of scores applicable to other series. We believe that to do this, a larger sample size would be necessary, as well as external validation with a different sample. Other limitations relate to a single-center register and the partial availability of factors such as neuron-specific enolase, electroencephalograms, and inflammatory markers.
It is important to highlight that this early prognostic approach must not under any circumstances replace a detailed multidisciplinary evaluation once sedation has been withdrawn, as decisions regarding maintenance of the therapeutic effort should be made carefully and be based on parameters with near-100% specificity. The primordial objective of this study was to illustrate a simple and quick way of making an early neurological evaluation in this situation. Given the routine sequence of actions (induction-maintenance-rewarming-withdrawal of sedation), information is usually scarce in the first 72hours and our findings can complement a later evaluation and improve the information for the patient's family, especially when neurological recovery seems likely.