Cardiac allograft vasculopathy affects both epicardial and microcirculatory coronary compartments. Magnetic resonance perfusion imaging has been proposed as a useful tool to assess microcirculation mostly outside the heart transplantation setting. Instantaneous hyperemic diastolic flow velocity-pressure slope, an intracoronary physiology index, has demonstrated a better correlation with microcirculatory remodelling in cardiac allograft vasculopathy than other indices such as coronary flow velocity reserve. To investigate the potential of magnetic resonance perfusion imaging to detect the presence of microcirculatory remodeling in cardiac allograft vasculopathy, we compared magnetic resonance perfusion data with invasive intracoronary physiological indices to study microcirculation in a population of heart transplantation recipients with macrovascular nonobstructive disease demonstrated with intravascular ultrasound.
MethodsWe studied 8 heart transplantation recipients (mean age, 61 [12] years, 100% male) with epicardial allograft vasculopathy defined by intravascular ultrasound, nonsignificant coronary stenoses and negative visually-assessed wall-motion/perfusion dobutamine stress magnetic resonance. Quantitative stress and rest magnetic resonance perfusion data to build myocardial perfusion reserve index, noninvasively, and 4 invasive intracoronary physiological indices were determined.
ResultsPostprocessed data showed a mean (standard deviation) myocardial perfusion reserve index of 1.22 (0.27), while fractional flow reserve, coronary flow velocity reserve, hyperemic microvascular resistance and instantaneous hyperemic diastolic flow velocity-pressure slope were 0.98 (0.02), cm/s/mmHg, 2.34 (0.55) cm/s/mmHg, 2.00 (0.69) cm/s/mmHg and 0.91 (0.65) cm/s/mmHg, respectively. The myocardial perfusion reserve index correlated strongly only with the instantaneous hyperemic diastolic flow velocity-pressure slope (r=0.75; P=.033).
ConclusionsMyocardial perfusion reserve index derived from a comprehensive dobutamine stress magnetic resonance appears to be a reliable technique for noninvasive detection of microcirculatory coronary disease associated with cardiac allograft vasculopathy.
Keywords
Heart transplantation (HT) is an effective treatment for selected patients with end-stage heart failure.1,2 Cardiac allograft vasculopathy (CAV) is the leading cause of late death among HT recipients.3 Epicardial coronary arteries as well as microvasculature (microvascular allograft vasculopathy) are affected in CAV.
Both obstructive and nonobstructive (microvascular) components of CAV influence patient prognosis.4 Detection of CAV is clinically relevant in the management of HT recipients. Immune-modulatory therapy modification is recommendable once CAV is detected because several immunosuppressive drugs have been shown to slow CAV progression and reduce events related to this entity.5–7
Coronary intravascular ultrasound is currently considered the gold standard for detection of macrovascular allograft vasculopathy. Due to the peculiar concentric distribution of CAV, detection of macrovascular allograft vasculopathy is higher by intravascular ultrasound than by angiography because an important subset of patients with apparently normal angiograms is affected by CAV.8–10 Intravascular ultrasound also provides important prognostic information8 and has therefore been established as the routine technique for the detection of macrovascular allograft vasculopathy in experienced HT centers. Despite the usefulness of intravascular ultrasound, this technique does not explore the microvasculature and is therefore not useful to diagnose microvascular allograft vasculopathy.
Assessment of microvascular allograft vasculopathy is hampered by the lack of a robust methodology. It has been customary to assess microvascular allograft vasculopathy using the intracoronary Doppler wire to measure coronary flow velocity reserve (CFVR).11,12 However, our group recently reported that combined pressure and flow velocity indices, particularly the instantaneous hyperemic flow pressure velocity slope (IHDVPS) index, correlate better with structural microcirculatory remodeling documented in cardiac biopsies than CFVR.13
Cardiac magnetic resonance (MR) provides a comprehensive evaluation of cardiac structure and function, allowing assessment of complete segmental wall motion and myocardial perfusion. Dobutamine stress MR has been proven to be useful for the detection of stenosis in epicardial coronary arteries.14 Visual assessment of dobutamine stress MR perfusion imaging has also been applied for the study of microvasculature in X syndrome.15 Although without invasive intracoronary validation, quantitative myocardial perfusion indices have also been developed and applied to the study of the microcirculatory component of CAV.16 Use of comprehensive cardiac stress MR to explore both, macro- and microvascular components of the coronary tree in HT patients has not been previously evaluated.
The aim of this study was to evaluate the value of noninvasive stress perfusion MR to detect microvascular allograft vasculopathy, using the IHDVPS as an invasive reference index.
METHODSSubjectsSeventeen consecutive clinically stable HT recipients with CAV diagnosed by intravascular ultrasound as harboring at least 1 coronary segment with Stanford classification ≥ class 3 were included. The mean (standard deviation) time from HT to the MR study was 10.3 (5.47) years. To avoid interference of epicardial stenoses on coronary hemodynamics, the study was conducted in cardiac allografts without obstructive coronary disease, assessed angiographically and confirmed with intracoronary physiology. Therefore none of the patients had significant stenosis in epicardial arteries on angiography. To assure a “functionally pure scenario” to study the microvascular compartment, we included only participants who were classified as negative on a visually–assessed dobutamine stress MR. Thus, all patients showed negative wall-motion and negative visually-assessed perfusion on dobutamine stress MR. The study was approved by our institution's local review board and conformed to the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants.
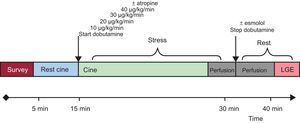
Dobutamine Stress Magnetic ResonanceThe studies were performed with a 1.5 T scanner (Philips Achieva® CV; Best, The Netherlands) equipped with a gradients PowerTrak 6000 system (23 mT/m, rise time 219μs; Philips Achieva®). Cardiac synchronization was performed with 4 electrodes in the anterior region of the left hemithorax. For cine imaging sequences, balanced steady-state free precession was used with retrospective cardiac gating. Standard cardiac geometries were acquired (3 short-axis: basal, mid and apical, and 3 long-axis: 4-chamber, 2-chamber and 3-chamber) both at rest and at every step of the stress protocol (Figure 1).
Dobutamine was infused using a Space Perfusor® pump (B. Braun Melsungen AG; Germany) at a standard increasing rate, from 10 to 40 μg/kg/min (Figure 1). Target heart rate = (theoretical maximum heart rate = 220 – age) × (0.85). Once the target heart rate was achieved, stress first-pass perfusion imaging was performed and dobutamine infusion was stopped immediately after. Although adenosine could have been used as a pharmacologic stressor, high specificity for wall motion abnormality described on dobutamine stress MR17 was preferred. For myocardial perfusion imaging, steady-state free precession sequences were used with the following parameters: echo time, 1.4ms; repetition time, 2.8ms; and flip angle, 50°, along with a saturation prepulse, acquiring 3 short-axis per beat. The spatial resolution was 2.8×3×10mm. SENSE factor 3.0 was used. Gadobutrol was administered (Gadovist ® Bayer Schering Pharma; Berlin, Germany) through a peripheral vein at a dose of 0.1 mmol/kg for first-pass myocardial perfusion at a rate of 3mL/s, using a pump COVIDIEN Optistar™ LE MR Injector (Siemens; Munich, Germany). Following a 10-min wait to allow a heart rate of around 100 bpm, a new dose of 0.1 mmol/kg was infused intravenously at a rate of 3mL/s to complete the total 0.2 mmol/kg dose used to study myocardial perfusion at rest. Standard late gadolinium enhancement imaging was performed18 (an example of dobutamine stress MR is shown in Figure 1).
Stress ProtocolWall Motion and Qualitative Magnetic Resonance Perfusion AnalysisAll image analysis was performed using the workstation Extended Workspace® (Philips Medical Systems; Best, The Netherlands). Two observers, blinded to clinical, perfusion, angiographic and physiology data, performed segmental wall analysis using a synchronized quad-screen image display and applying a standard 16-segment method.
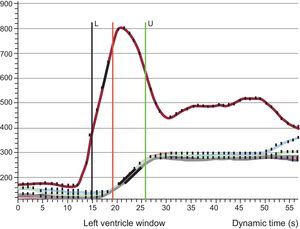
Quantitative Perfusion AnalysisThe myocardial perfusion reserve index (MPRI) was defined as the ratio of hyperemic and basal myocardial blood flow. Therefore, the MPRI was calculated as the ratio of the normalized time-signal upslopes measured for rest and stress images.19,20 Upslope was defined as the first derivative of the time-intensity curve during the ascent of the first passes; units were signal intensity per second (S/s). The upslope of the myocardium was normalized by the upslope of left ventricular blood pool to correct for differences of the speed and compactness of the contrast agent bolus.21,22 Stress and rest upslopes were measured in segment 8 (mid anteroseptal) according to 16-segment nomenclature23 to better assess myocardial tissue from left anterior descending artery territory (Doppler-wire measurements are performed in the midleft anterior descending artery). An example of the time-intensity signal curve is shown in Figure 2.
Perfusion imaging postprocessing. Time-intensity-curve of the blood pool (red line) and 6 segments in the midventricle (overlapping lines). The upslope was calculated and corrected with blood pool (see text for details). L, lower frame included in the analysis; U, upper frame included in the analysis.
A standard catheterization procedure was performed. Two experienced cardiologists analyzed coronary angiography data independently and blinded to dobutamine stress MR data. Significant coronary stenosis was defined as a lumen stenosis ≥ 70% in at least 1 of the coronary arteries or their major branches. Intravascular ultrasound images were acquired using 40MHz Atlantis intravascular ultrasound catheters and the corresponding Galaxy2® console (Boston Scientific; Massachusetts, United States), which also served as a workstation for intravascular ultrasound measurements. Slow pullback of 2 major epicardial coronary arteries, ie, left anterior descending artery and circumflex artery, towards their ostia was performed. The position of the transducer was angiographically determined. Coronary segments were defined according to standard anatomical landmarks. Representative images were acquired for each segment. The most affected area was selected in each segment and was quantitatively evaluated according to the degree of thickness and circumferential extent of intimal hyperplasia. For CAV evaluation, we used the Stanford classification and the index of intimal thickening as previously described.24,25
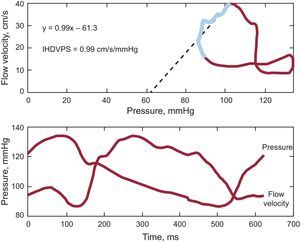
Intravascular HemodynamicsCoronary flow measurements were performed in the midsegment of the left anterior descending artery using an 0.014” intracoronary guidewire fitted with Doppler and pressure sensors (ComboMap®, Volcano Corporation; San Diego, California, United States) connected to the corresponding interface (ComboMap, Volcano Corporation, San Diego, California, United States). Measurements were obtained 3 min to 5 min after the intracoronary administration of nitroglycerin for the rest phase and afterward repeated measurements were taken during hyperemia with adenosine for the stress phase. Digital electrocardiogram, aortic pressure and instantaneous intracoronary peak flow data were extracted from the console and analyzed using a custom software package designed with MATLAB® (Mathworks, Inc.; Natick, Massachusetts, United States). The following indices were calculated: a) fractional flow reserve26: as the ratio of distal coronary pressure (Pd) to proximal coronary pressure (Pa) at maximal hyperemia; b) CFVR26: as the ratio between the average of peak velocities (Vcor) measured at rest and during hyperemia (Vcor hyperemia/Vcor basal); c) coronary resistance reserve: as the ratio between resting coronary resistance (Pd / Vcor basal) and hyperemic coronary resistance (Pd / Vcor at hyperemia); d) hyperemic microvascular resistance27: as the ratio between mean aortic pressure (Pa) and average peak velocities during hyperemia (Pa / Vcor at hyperemia), and finally; e) the slope of the IHDVPS defined as the slope of the pressure-velocity flow during mid- and end-diastole under maximal hyperemia.28 Diastolic pressure and flow rate measurements were automatically identified using as reference peak flow velocity (initiation of middiastole) and the fast decrease in diastolic velocity at the end of diastole. Linear regression analysis was applied to the selected data and a slope of the regression curve (diastolic coronary conductance under hyperemia) expressed in mmHg/cm/s was obtained. The linearity of the relationship in this specific range was described using the regression coefficient r2. A IHDPVS calculation example is shown in Figure 3.
Coronary flow velocity/pressure relationship in one of the patients included in the study. Upper panel shows the pressure/flow velocity loop obtained by averaging several beats during maximal hyperemia. A instantaneous hyperemic diastolic velocity pressure slope index of 0.9mmHg/cm/s was calculated by performing linear regression analysis (dotted line) of values measured in mid- and late-diastole (blue segment in loop). The lower panel shows pressure and flow velocity waveforms. IHDVPS, instantaneous hyperemic diastolic flow velocity-pressure slope.
The statistical package used was IBM SPSS Statistics, release 20.0.0. Continuous variables were expressed as mean (standard deviation). Discrete data are shown as the number of participants and %. Person correlation was used to compare continuous variables. Statistical tests were bilateral; significance was reached if P<.05.
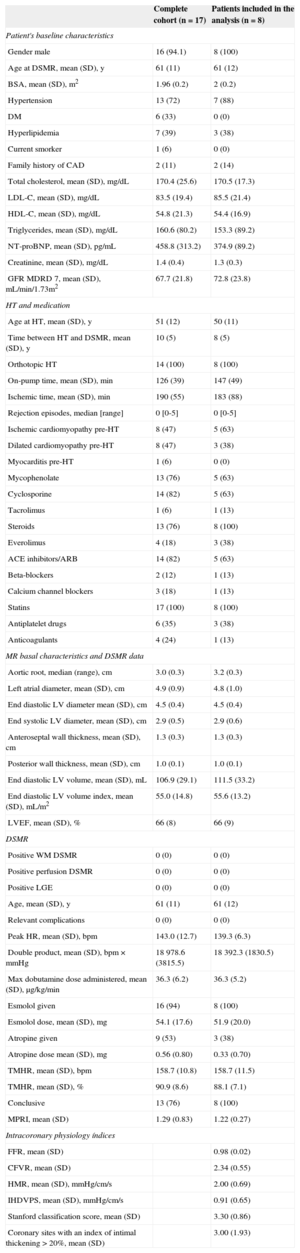
RESULTSSeventeen HT recipients (61 [11] years old, 16 males, time from transplant 10 [5] years) were initially recruited. Three patients were excluded from analysis due to the lack of either dobutamine stress MR (1 patient, claustrophobia) or catheterization procedure (2 patients: traumatic death and aortic thrombus), and 4 patients because of inconclusive stress conditions to evaluate MPRI. Finally 2 patients were excluded due to technical issues in invasive microcirculatory assessment. Therefore, 8 patients were finally eligible for the noninvasive vs invasive comparison performed in the study. The patients’ baseline characteristics and MR data are shown in the Table.
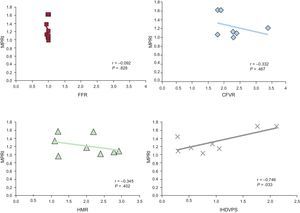
In the perfusion postprocessing analysis, the mean (standard deviation) MPRI value measured at segment 8 was 1.22 (0.27). Intracoronary measurements yielded the following values: fractional flow reserve 0.98 (0.02) (confirming the absence of obstructive epicardial disease); CFVR, 2.34 (0.55) (in 1 patient CFVR could not be calculated due to artifacts in the recorded baseline flow velocity); hyperemic microvascular resistance, 2.00 (0.69) cm/s/mmHg, and IHDVPS, 0.91 (0.65) cm/s/mmHg. In investigating the relationship between these intracoronary indices and MPRI, a significant correlation was documented for IHDVPS (r=0.75; P=.033), but not for fractional flow reserve, CFVR or hyperemic microvascular resistance (Table, Figure 4).
Patient Characteristics
| Complete cohort (n=17) | Patients included in the analysis (n=8) | |
|---|---|---|
| Patient's baseline characteristics | ||
| Gender male | 16 (94.1) | 8 (100) |
| Age at DSMR, mean (SD), y | 61 (11) | 61 (12) |
| BSA, mean (SD), m2 | 1.96 (0.2) | 2 (0.2) |
| Hypertension | 13 (72) | 7 (88) |
| DM | 6 (33) | 0 (0) |
| Hyperlipidemia | 7 (39) | 3 (38) |
| Current smorker | 1 (6) | 0 (0) |
| Family history of CAD | 2 (11) | 2 (14) |
| Total cholesterol, mean (SD), mg/dL | 170.4 (25.6) | 170.5 (17.3) |
| LDL-C, mean (SD), mg/dL | 83.5 (19.4) | 85.5 (21.4) |
| HDL-C, mean (SD), mg/dL | 54.8 (21.3) | 54.4 (16.9) |
| Triglycerides, mean (SD), mg/dL | 160.6 (80.2) | 153.3 (89.2) |
| NT-proBNP, mean (SD), pg/mL | 458.8 (313.2) | 374.9 (89.2) |
| Creatinine, mean (SD), mg/dL | 1.4 (0.4) | 1.3 (0.3) |
| GFR MDRD 7, mean (SD), mL/min/1.73m2 | 67.7 (21.8) | 72.8 (23.8) |
| HT and medication | ||
| Age at HT, mean (SD), y | 51 (12) | 50 (11) |
| Time between HT and DSMR, mean (SD), y | 10 (5) | 8 (5) |
| Orthotopic HT | 14 (100) | 8 (100) |
| On-pump time, mean (SD), min | 126 (39) | 147 (49) |
| Ischemic time, mean (SD), min | 190 (55) | 183 (88) |
| Rejection episodes, median [range] | 0 [0-5] | 0 [0-5] |
| Ischemic cardiomyopathy pre-HT | 8 (47) | 5 (63) |
| Dilated cardiomyopathy pre-HT | 8 (47) | 3 (38) |
| Myocarditis pre-HT | 1 (6) | 0 (0) |
| Mycophenolate | 13 (76) | 5 (63) |
| Cyclosporine | 14 (82) | 5 (63) |
| Tacrolimus | 1 (6) | 1 (13) |
| Steroids | 13 (76) | 8 (100) |
| Everolimus | 4 (18) | 3 (38) |
| ACE inhibitors/ARB | 14 (82) | 5 (63) |
| Beta-blockers | 2 (12) | 1 (13) |
| Calcium channel blockers | 3 (18) | 1 (13) |
| Statins | 17 (100) | 8 (100) |
| Antiplatelet drugs | 6 (35) | 3 (38) |
| Anticoagulants | 4 (24) | 1 (13) |
| MR basal characteristics and DSMR data | ||
| Aortic root, median (range), cm | 3.0 (0.3) | 3.2 (0.3) |
| Left atrial diameter, mean (SD), cm | 4.9 (0.9) | 4.8 (1.0) |
| End diastolic LV diameter mean (SD), cm | 4.5 (0.4) | 4.5 (0.4) |
| End systolic LV diameter, mean (SD), cm | 2.9 (0.5) | 2.9 (0.6) |
| Anteroseptal wall thickness, mean (SD), cm | 1.3 (0.3) | 1.3 (0.3) |
| Posterior wall thickness, mean (SD), cm | 1.0 (0.1) | 1.0 (0.1) |
| End diastolic LV volume, mean (SD), mL | 106.9 (29.1) | 111.5 (33.2) |
| End diastolic LV volume index, mean (SD), mL/m2 | 55.0 (14.8) | 55.6 (13.2) |
| LVEF, mean (SD), % | 66 (8) | 66 (9) |
| DSMR | ||
| Positive WM DSMR | 0 (0) | 0 (0) |
| Positive perfusion DSMR | 0 (0) | 0 (0) |
| Positive LGE | 0 (0) | 0 (0) |
| Age, mean (SD), y | 61 (11) | 61 (12) |
| Relevant complications | 0 (0) | 0 (0) |
| Peak HR, mean (SD), bpm | 143.0 (12.7) | 139.3 (6.3) |
| Double product, mean (SD), bpm × mmHg | 18978.6 (3815.5) | 18392.3 (1830.5) |
| Max dobutamine dose administered, mean (SD), μg/kg/min | 36.3 (6.2) | 36.3 (5.2) |
| Esmolol given | 16 (94) | 8 (100) |
| Esmolol dose, mean (SD), mg | 54.1 (17.6) | 51.9 (20.0) |
| Atropine given | 9 (53) | 3 (38) |
| Atropine dose mean (SD), mg | 0.56 (0.80) | 0.33 (0.70) |
| TMHR, mean (SD), bpm | 158.7 (10.8) | 158.7 (11.5) |
| TMHR, mean (SD), % | 90.9 (8.6) | 88.1 (7.1) |
| Conclusive | 13 (76) | 8 (100) |
| MPRI, mean (SD) | 1.29 (0.83) | 1.22 (0.27) |
| Intracoronary physiology índices | ||
| FFR, mean (SD) | 0.98 (0.02) | |
| CFVR, mean (SD) | 2.34 (0.55) | |
| HMR, mean (SD), mmHg/cm/s | 2.00 (0.69) | |
| IHDVPS, mean (SD), mmHg/cm/s | 0.91 (0.65) | |
| Stanford classification score, mean (SD) | 3.30 (0.86) | |
| Coronary sites with an index of intimal thickening > 20%, mean (SD) | 3.00 (1.93) | |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BSA, bovine serum albumin; CAD, coronary artery disease; CFVR, coronary flow velocity reserve; DM, diabetes mellitus; DSMR, dobutamine stress magnetic resonance; FFR, fractional flow reserve; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HMR, hyperemic microvascular reserve; HR, heart rate; HT, heart transplantation; IHDVPS, instantaneous hyperemic diastolic flow velocity-pressure slope; LDL-C, low-density lipoprotein cholesterol; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; MPRI, myocardial perfusion reserve index; MR, magnetic resonance; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SD, standard deviation;TMHR, theoretical maximum heart rate; WM, wall motion.
Data are expressed as No. (%), mean (standard deviation) or median [range].
Pearson correlations between intracoronary physiology indices. CFVR, coronary flow velocity reserve; FFR, fractional flow reserve; HMR, hyperemic microvascular resistance; IHDVPS, instantaneous hyperemic diastolic flow velocity-pressure slope; MPRI, myocardial reserve perfusion index. Pearson correlation coefficient r, and P value are shown for each comparison.
All patients included in the analysis had evidence of macrovascular allograft vasculopathy in intravascular ultrasound with, as per protocol, at least 1 coronary site of Stanford classification class ≥ 3.
DISCUSSIONThe main finding of our study is that, in patients with cardiac allografts, noninvasive quantitative assessment of myocardial perfusion with MR imaging reflects the presence of underlying microcirculatory disease associated with structural remodelling, as evaluated by intracoronary assessment of microcirculatory hemodynamics. To strengthen these observations, the study was conducted in a selected population of patients with intravascular ultrasound-detected CAV but no obstructive epicardial disease.
The availability of methods to assess the status of coronary microcirculation remains a pending subject in adequately assessing the prognosis of ischemic heart disease in atherosclerosis29 and other conditions such as CAV.4 The potential use of MR to noninvasively evaluate the presence and transmural distribution of microcirculatory disease was first revealed in the assessment of acute coronary syndromes,30 with subsequent studies revealing its prognostic implications.31
Several studies have evaluated MPRI in several microvascular disorders, although CFVR has usually been taken as the intracoronary invasive reference.15,16 In 2003, Muehling et al.16 assessed for the first time CAV (both macrovascular allograft vasculopathy and microvascular allograft vasculopathy) with MPRI, using angiography and CFVR as references. This group reported that a cutoff point for MPRI of<2.3 had a sensitivity and specificity of 100% for the detection of CAV. In that study, a CFVR cutoff value of<2.5 was used to define microvascular allograft vasculopathy. More recently, Lanza et al15 demonstrated the ability of perfusion MR to evaluate coronary microcirculation in cardiac syndrome X, a condition in which microcirculatory dysfunction allegedly plays a major role. This group highlighted the concurrence of myocardial perfusion defects (assessed visually) and a reduced value of CFVR in affected individuals.
In the present study, we addressed the value of MR perfusion sequences to quantify the degree of microvascular allograft vasculopathy in transplant patients. Unlike previous studies, the study population was carefully selected to ensure that the presence of evident or concealed obstructive coronary disease would not interfere with the observations. Patients were thus carefully selected and underwent multiple testing at the expense of limiting the population size. First, the presence of obstructive epicardial disease that might influence the observations at a microcirculatory level was excluded not only by limiting inclusion to cardiac allografts without angiographic stenoses, but also by demonstrating a normal epicardial conductance with fractional flow reserve. The use of physiology to confirm angiographic findings is important, since diffuse luminal irregularities may cause significant intracoronary pressure loss in the absence of focal stenoses.32 In this regard, it is worth noting that fractional flow reserve values in our series (0.98 [0.02]) were similar to those reported by Melikian et al33 in a population of control participants without coronary atheroma (0.96 [0.02]). Second, intravascular ultrasound was performed to document nonobstructive evidence of allograft vasculopathy, demonstrating a Stanford score of 3.30 (0.86). Finally, a negative wall-motion analysis for dobutamine stress MR was a prerequisite for study inclusion.
Furthermore, an alternative intracoronary physiology index to CFVR was used as a reference to assess the presence of microvascular allograft vasculopathy. This was required because, despite having been frequently used to evaluate microcirculatory status, CFVR is limited by being a relative index of coronary flow. This implies that any modification of baseline flow velocity resulting from hemodynamic factors, age, diabetes mellitus or sex, to name a few factors, will affect the final CFVR value.34 This may potentially be more relevant in cardiac allografts, since denervation and enhanced sympathomimetic tone may influence baseline flow velocity measurements.
Due to these considerations, in a previous work we explored the possibility of using baseline flow-independent indices based on either whole-cycle microcirculatory resistance (coronary resistance index) or on the coronary pressure-flow velocity relationship measured selectively during mid- and late-diastole IHDVPS. Specifically, IHDVPS demonstrated an excellent, independent correlation with 2 important elements of structural microcirculatory remodeling in CAV namely, arteriolar obliteration, and capillary rarefaction.13 When the combined contribution of these 2 elements of microcirculatory disease was taken into account, the strength of the relationship between IHDVPS and the underlying microcirculatory changes (r=0.84; P=.0002) was far superior to CFVR and to the microcirculatory resistance ratio.13
Although both indexes are derived from the same theoretical framework, namely coronary flow reserve, in our study, we could not document a significant relationship between MPRI and CFVR. In understanding this discrepancy, 2 important differences between these 2 indexes should be remembered: a) MPRI assesses changes in myocardial blood in response to stress, while CFVR assesses changes in flow velocity in an epicardial artery, and b) maximal hyperemia is obtained in response to dobutamine administration in MPRI, while intravenous adenosine infusion is used in CFVR; although intravenous dobutamine infusion has been found to be as effective as adenosine at dosages of 40 μg/kg/min in inducing myocardial hyperemia,35 in practice this dosage is not always achievable, as demonstrated in our series (in 3 patients [38%] the stress protocol had to be stopped before that dobutamine dose).
The MPRI values obtained in our study were similar to those registered in the so-called group C population published by Muehling et al.16 Both populations (group C of Muehling et al and ours) had a similar profile (macrovascular allograft vasculopathy was present and CFVR was relatively low).
On the other hand, the existence of a significant correlation between MPRI and IHDVPS, and not with CFVR, mirrors our previous comparisons of both indices with histomorphometric findings in cardiac biopsies of cardiac allografts, and provides a new piece of evidence supporting the value of HIDVPS for this purpose. It is also interesting to note that hyperemic microvascular resistance, a microcirculatory resistance index similar to the coronary resistance index used in our previous validation work, did not correlate significantly with MR findings. Because this resistance index is obtained from averaged, whole cardiac cycle measurements of pressure and flow velocity, hyperemic microvascular resistance may be not as sensitive as IHDVPS in the detection of microvascular allograft vasculopathy, while still being superior to CFVR.13
As shown in our study, MPRI had an excellent and statistically significant correlation with IHDVPS. According to the results of our analysis, dobutamine stress MR was a safe test in patients after HT if they were clinically stable. The safety profile of the technique is comparable to results previously published in non-HT patients.36 No extraordinary safety measures for these patients were necessary. Unlike published evidence in HT recipients with denerved hearts,37 a chronotropic response to atropine was found and therefore this drug would be considered useful for pharmacological stress protocols to reach the target heart rate.
To our knowledge, no validation of MR perfusion sequences with IHDVPS has previously been published. Potentially, our research would facilitate the development of a noninvasive platform, comprehensive dobutamine stress MR, as a model for study both vascular compartments, helping to consolidate MR as a valid approach for the evaluation of microcirculatory disease. In terms of clinical applicability, due to the intrinsic difficulties of routinely performing invasive tests in HT patients, our proposed noninvasive methodology, MPRI, could be an attractive alternative for the follow-up of the CAV, given its high accuracy for the identification of micro- and macrovascular allograft vasculopathy. If CAV is detected early in these patients, improvements in clinical outcomes could be made through adequate medication management.5,6
Study LimitationsThe most obvious limitation of our study is its small sample size, resulting from the complexity of the study and the rigorous patient selection made to ensure the validity of our observations. Different pharmacologic stressors were used for each technique (adenosine for the calculation of IHDVPS and dobutamine for MPRI). Although the stress generated on both tests appeared to be equal and this approach has been used in the past,15 we cannot guarantee there are no differences derived from using different drugs. Although perfusion analysis was performed at segment 8 (midanteroseptal) because the Doppler-wire was positioned in the midleft anterior descending artery, evaluation of alternative segments (including those supplied by the circumflex or right coronary artery) should be assessed in specific protocols.
CONCLUSIONSThe quantitative, fully available MR perfusion index comparing stress/rest time-intensity-curves upslopes, MPRI, had an excellent correlation with a histologically validated Doppler-wire intracoronary index for the detection of microvascular allograft vasculopathy in a functionally pure microvascular scenario (nonsignificant coronary stenosis at angiography and negative wall-motion at dobutamine stress MR). We propose MPRI for the study and follow-up of HT patients for the detection and repercussion of microvascular allograft vasculopathy.
FUNDINGThis work was partially supported by a grant from the Spanish Society of Cardiology (Clinical Research in Cardiology, 2009) and, the Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid (Spain) (CardioImagen to J.G.M.) and the Spanish Ministry of Health (RD12/0042/0066).
CONFLICTS OF INTERESTJ. Escaned has served as speaker in educational activities organized by Boston Scientific, St Jude Medical and Volcano Corporation.
We gratefully acknowledge our technicians for their excellent technical assistance during cardiac stress MR examinations and also Borja Ibañez PhD from Centro Nacional de Investigaciones Cardiovaculares (CNIC), Justin Davies from the Imperial College Healthcare and NHS Trust, London (United Kingdom), and Rolf Gebker from the German Heart Institute, Berlin (Germany) for their review of the manuscript and support of the project.