Invasive and pharmacological treatment of ST-segment elevation acute myocardial infarction reduces the rate of ischemic events but not bleeding complications. The objective of this study was to compare clinical results and bleeding complications between femoral and radial access routes in patients with ST-segment elevation acute myocardial infarction.
MethodsAn evaluation was performed of the population of the Examination study, a randomized, multicenter, clinical trial that included 1498 patients with ST-segment elevation acute myocardial infarction who underwent emergency angioplasty. Subanalysis of this population was conducted to compare patients by type of access (femoral vs radial). The primary end point was a composite of: all-cause death, myocardial infarction, revascularization, and bleeding.
ResultsFemoral and radial access routes were used in 825 (55%) and 673 (45%) patients, respectively. More bleeding complications (major and minor) were seen with femoral access than radial access (5.9% vs 2.8%; P<.004), largely due to a greater incidence of minor bleeding with femoral access (4.6% vs 1.9%; P=.005). After adjustment for confounders, survival analysis showed a reduction in the primary composite end point in patients with radial access (hazard ratio=0.73; 95% confidence interval, 0.56-0.96; P=.022).
ConclusionsIn patients with ST-segment elevation acute myocardial infarction, the radial approach is an effective technique that improves prognosis and reduces bleeding complications.
Keywords
The femoral artery has been the approach of choice in 44.5% to 93.1% of invasive procedures in recent reports.1,2 This access route is frequently chosen because the radial approach requires proper patient selection and extensive operator experience and has a steep learning curve, particularly for ST-segment elevation myocardial infarction (STEMI).3,4 The most common complication in patients with acute coronary syndrome treated with angioplasty is bleeding, which is often associated with vascular access. Bleeding is associated with greater inhospital mortality and worse prognosis.5–7 In the current study, the clinical results and bleeding complications of the femoral approach were compared with those of the radial approach in consecutive patients included in the Examination study who underwent coronary angioplasty for STEMI.8
METHODSStudy PopulationThe Examination study is a prospective, 1:1 randomized, controlled, and multinational 12-center clinical trial that included consecutive patients with STEMI referred for emergency coronary angioplasty. Patients were included from 31 December 2008 to 15 May 2010. The electrocardiographic criteria of STEMI were ST-segment elevation ≥ 1mm in 2 or more standard leads or ≥ 2mm in 2 or more standard contiguous precordial leads or unknown left bundle branch block within the first 48h after symptom onset. The study was designed to compare clinical results between everolimus drug-eluting stents (Xience V, Abbott Vascular; Santa Clara, California, United States) and expandable cobalt chromium drug-free Multilink Vision stents (Abbott Vascular). The methodology and clinical results of the first year were recently published.8 This study was approved by the respective clinical research ethics committees of the participating hospitals and all patients provided written informed consent. For this randomized study, an unplanned cohort subanalysis was performed by establishing 2 groups according to approach: femoral vs radial.
ProcedureAll patients admitted with STEMI for emergency angioplasty received anticoagulant therapy and antiplatelet agents according to hospital protocol. A loading dose of acetylsalicylic acid (250-500mg) and clopidogrel (300-600mg) was administered before the procedure to patients who were not on chronic antiplatelet therapy. Anticoagulant therapy was performed with unfractionated or low-molecular-weight heparin. Bivalirudin or inhibitors of platelet glycoprotein IIb/IIIa receptors were used at the discretion of the catheterization cardiologist. Manual thrombectomy, followed by direct stenting, was the recommended revascularization strategy. The type of stent was randomly assigned by telephone call to the coordinating center. Patients were discharged with dual antiplatelet agent therapy: clopidogrel (75 mg/day) for at least 1 year and acetylsalicylic acid (100 mg/day) indefinitely. Data were analyzed by an independent CoreLab (Cardialysis BV; Rotterdam, The Netherlands).
Definitions and Follow-upThe primary end point of this study was a composite of: all-cause death, myocardial infarction, revascularization of the target vessel, and major or minor bleeding.9 Two groups were defined according to use of a femoral or radial vascular approach.
Major cardiac events were defined as all-cause death, myocardial infarction, and revascularization of the target vessel, in agreement with the Academic Research Consortium.10 Stent thrombosis was defined as “definite” when there was angiographic or autopsy confirmation; “probable” when there was sudden unexplained death within 30 days after the intervention or a documented infarction in the territory of the treated artery, and “possible” for all sudden unexplained death that occurred 30 days after the procedure.
Bleeding complications were defined as major bleeding when there was a decrease in hemoglobin values ≥ 5g/dL, a decrease in the hematocrit ≥ 15%, intracranial bleeding, or any bleeding associated with hemodynamic alterations that required blood transfusion. Minor bleeding was defined as a decrease in hemoglobin values of 3 to 5g/dL or in hematocrit of 12% to 15%, regardless of source.11
Follow-up was conducted via clinical visits or by telephone at 30 days, 6 months, and 1 year, and will continue until 5 years have passed.
Statistical AnalysisClinical and baseline anatomical variables were tabulated, as well as those related to the procedure and clinical events during follow-up. A Student t test or Wilcoxon test was used to detect differences between continuous variables. A chi-square or Fisher exact test was used for categorical variables. The probability of event-free survival of the composite end point depending on type of vascular access was calculated using Kaplan-Meier survival analysis and a log rank test. A Cox proportional hazards model was used to identify independent predictive variables of the primary composite end point. A univariate analysis was performed to select variables to enter in the Cox analysis as predictors of the primary composite end point: only those variables with P<.01 were considered possible confounding factors (smoking, diabetes mellitus, hypertension, single-vessel disease, angioplasty and previous myocardial infarction, Killip class on admission, ejection fraction on discharge, and radial access use), age, sex, and extensive experience in the use of the radial approach (centers where more than 90% of cases were performed by radial access). Age and ejection fraction were entered as continuous variables, sex coded as female, and Killip class as class IV vs other classes. The other variables were entered as dichotomous variables, coding 1 as presence and 0 as absence. The significance of the resulting Cox model was tested and the variables were ranked. In addition, interactions between bleeding and other events in the primary composite end point were analyzed by repeating the survival analysis without the bleeding variable and the first-degree interaction between the type of stent implant and the type of arterial access. Results are expressed in absolute values, percentages, and mean (2 standard deviations). All comparisons were bilateral and were considered significant at P<.05. SPSS version 21 was used for all analyses.
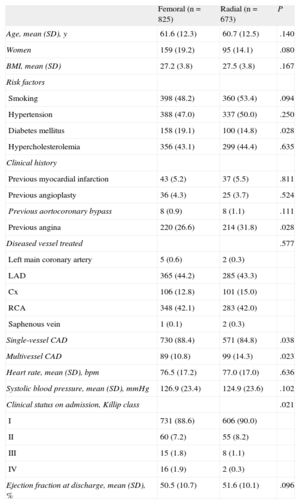
RESULTSClinical and Anatomical CharacteristicsAngioplasty was performed for STEMI in 1498 patients; access was via the femoral approach in 825 patients (55%) and via the radial approach in the remaining 673 patients (45%). The baseline clinical and anatomical characteristics are described in Table 1. No significant differences were seen between the patients that were operated on using femoral access vs radial access for age, sex, body surface, coronary risk factors (smoking, hypertension, and hypercholesterolemia), and history of revascularization. Access was largely femoral in patients with diabetes mellitus (P=.028) and with worse clinical status on admission (P=.021), and mainly radial in patients with previous angina (P=.028) and with multivessel heart disease (P=.023). Of the centers that participated in the study, 4 used the radial approach in > 90% of the included patients. In the other centers, the mean use of the radial approach was 18%. Of the 1498 randomized patients, 1460 (97%) had a 1-year follow-up.
Baseline Clinical and Anatomical Characteristics by Access Route
| Femoral (n=825) | Radial (n=673) | P | |
| Age, mean (SD), y | 61.6 (12.3) | 60.7 (12.5) | .140 |
| Women | 159 (19.2) | 95 (14.1) | .080 |
| BMI, mean (SD) | 27.2 (3.8) | 27.5 (3.8) | .167 |
| Risk factors | |||
| Smoking | 398 (48.2) | 360 (53.4) | .094 |
| Hypertension | 388 (47.0) | 337 (50.0) | .250 |
| Diabetes mellitus | 158 (19.1) | 100 (14.8) | .028 |
| Hypercholesterolemia | 356 (43.1) | 299 (44.4) | .635 |
| Clinical history | |||
| Previous myocardial infarction | 43 (5.2) | 37 (5.5) | .811 |
| Previous angioplasty | 36 (4.3) | 25 (3.7) | .524 |
| Previous aortocoronary bypass | 8 (0.9) | 8 (1.1) | .111 |
| Previous angina | 220 (26.6) | 214 (31.8) | .028 |
| Diseased vessel treated | .577 | ||
| Left main coronary artery | 5 (0.6) | 2 (0.3) | |
| LAD | 365 (44.2) | 285 (43.3) | |
| Cx | 106 (12.8) | 101 (15.0) | |
| RCA | 348 (42.1) | 283 (42.0) | |
| Saphenous vein | 1 (0.1) | 2 (0.3) | |
| Single-vessel CAD | 730 (88.4) | 571 (84.8) | .038 |
| Multivessel CAD | 89 (10.8) | 99 (14.3) | .023 |
| Heart rate, mean (SD), bpm | 76.5 (17.2) | 77.0 (17.0) | .636 |
| Systolic blood pressure, mean (SD), mmHg | 126.9 (23.4) | 124.9 (23.6) | .102 |
| Clinical status on admission, Killip class | .021 | ||
| I | 731 (88.6) | 606 (90.0) | |
| II | 60 (7.2) | 55 (8.2) | |
| III | 15 (1.8) | 8 (1.1) | |
| IV | 16 (1.9) | 2 (0.3) | |
| Ejection fraction at discharge, mean (SD), % | 50.5 (10.7) | 51.6 (10.1) | .096 |
BMI, body mass index; CAD, coronary artery disease; Cx, circumflex artery; LAD, left anterior descending artery; RCA, right coronary artery; SD, standard deviation.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
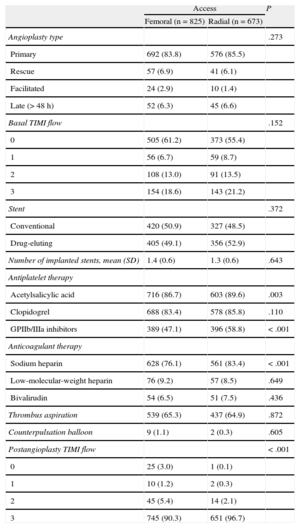
Variables related to the procedure and the antiplatelet and anticoagulant therapies are shown in Table 2. The number of primary, rescue, facilitated, or late (> 48h) angioplasties, basal TIMI (Thrombolysis In Myocardial Infarction) flow, the model and number of stents implanted, thrombus aspiration, and counterpulsation balloon implantation were similar between the 2 vascular access routes. Access was largely radial in patients pretreated with acetylsalicylic acid (P=.003), sodium heparin (P<.001), and inhibitors of platelet glycoprotein IIb/IIIa receptors (P<.001). Worse TIMI flow after angioplasty was seen in patients whose access was via the femoral approach (P<.001).
Procedure Characteristics
| Access | P | ||
| Femoral (n=825) | Radial (n=673) | ||
| Angioplasty type | .273 | ||
| Primary | 692 (83.8) | 576 (85.5) | |
| Rescue | 57 (6.9) | 41 (6.1) | |
| Facilitated | 24 (2.9) | 10 (1.4) | |
| Late (> 48 h) | 52 (6.3) | 45 (6.6) | |
| Basal TIMI flow | .152 | ||
| 0 | 505 (61.2) | 373 (55.4) | |
| 1 | 56 (6.7) | 59 (8.7) | |
| 2 | 108 (13.0) | 91 (13.5) | |
| 3 | 154 (18.6) | 143 (21.2) | |
| Stent | .372 | ||
| Conventional | 420 (50.9) | 327 (48.5) | |
| Drug-eluting | 405 (49.1) | 356 (52.9) | |
| Number of implanted stents, mean (SD) | 1.4 (0.6) | 1.3 (0.6) | .643 |
| Antiplatelet therapy | |||
| Acetylsalicylic acid | 716 (86.7) | 603 (89.6) | .003 |
| Clopidogrel | 688 (83.4) | 578 (85.8) | .110 |
| GPIIb/IIIa inhibitors | 389 (47.1) | 396 (58.8) | < .001 |
| Anticoagulant therapy | |||
| Sodium heparin | 628 (76.1) | 561 (83.4) | < .001 |
| Low-molecular-weight heparin | 76 (9.2) | 57 (8.5) | .649 |
| Bivalirudin | 54 (6.5) | 51 (7.5) | .436 |
| Thrombus aspiration | 539 (65.3) | 437 (64.9) | .872 |
| Counterpulsation balloon | 9 (1.1) | 2 (0.3) | .605 |
| Postangioplasty TIMI flow | < .001 | ||
| 0 | 25 (3.0) | 1 (0.1) | |
| 1 | 10 (1.2) | 2 (0.3) | |
| 2 | 45 (5.4) | 14 (2.1) | |
| 3 | 745 (90.3) | 651 (96.7) | |
GPIIb/IIIa, glycoprotein-IIb/IIIa; SD, standard deviation; TIMI, Thrombolysis In Myocardial Infarction.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
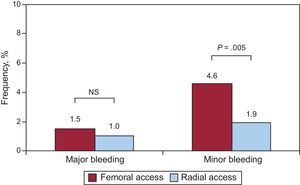
Bleeding (major/minor) was seen in 49 of the 825 patients operated on via femoral access (5.9%) and in 19 of the 673 patients operated on via radial access (2.8%; P<.004). The number of major bleeding cases was similar between femoral and radial approaches (Figure 1): major bleeding was seen in 13 patients who underwent a femoral approach (1.5%) and in 7 who underwent a radial approach (1.0%; P=.36). In contrast, minor bleeding was seen in 38 patients operated on via femoral access (4.6%) and in 13 operated on via radial access (1.9%; P=.005) (Figure 1).
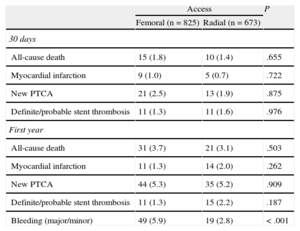
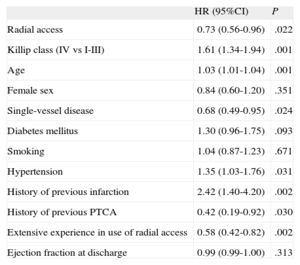
Clinical Events During Follow-up and Predictive FactorsThe incidence of stent thrombosis was independent of vascular approach. Moreover, there were no differences between the groups in the incidence of major cardiac events, all-cause death, myocardial infarction, or need for revascularization (Table 3). Kaplan-Meier analysis showed a greater number of events of the primary composite end point in patients with a femoral access, compared to radial access (hazard ratio [HR]=0.73); 95% confidence interval [95%CI], 0.56-0.96; P=.022), after adjusting for confounding factors (Figure 2). Independent risk factors of the primary composite end point are shown in Table 4, as identified by Cox regression analysis. The treatment variables of sodium heparin and administration of platelet glycoprotein IIb/IIIa receptor inhibitors were not predictive variables in the univariate model. There were no interactions between bleeding and other events in the primary composite end point, and there was no significant relationship in the survival model between the type of stent implanted and the type of arterial access.
Clinical Events During Follow-up
| Access | P | ||
| Femoral (n=825) | Radial (n=673) | ||
| 30 days | |||
| All-cause death | 15 (1.8) | 10 (1.4) | .655 |
| Myocardial infarction | 9 (1.0) | 5 (0.7) | .722 |
| New PTCA | 21 (2.5) | 13 (1.9) | .875 |
| Definite/probable stent thrombosis | 11 (1.3) | 11 (1.6) | .976 |
| First year | |||
| All-cause death | 31 (3.7) | 21 (3.1) | .503 |
| Myocardial infarction | 11 (1.3) | 14 (2.0) | .262 |
| New PTCA | 44 (5.3) | 35 (5.2) | .909 |
| Definite/probable stent thrombosis | 11 (1.3) | 15 (2.2) | .187 |
| Bleeding (major/minor) | 49 (5.9) | 19 (2.8) | < .001 |
PTCA, percutaneous transluminal coronary angioplasty.
Data are expressed as No. (%).
Predictors of the Composite End Point (Death, Infarction, Revascularization of the Treated Vessel, Bleeding) at 1 Year. Cox Regression Analysis
| HR (95%CI) | P | |
| Radial access | 0.73 (0.56-0.96) | .022 |
| Killip class (IV vs I-III) | 1.61 (1.34-1.94) | .001 |
| Age | 1.03 (1.01-1.04) | .001 |
| Female sex | 0.84 (0.60-1.20) | .351 |
| Single-vessel disease | 0.68 (0.49-0.95) | .024 |
| Diabetes mellitus | 1.30 (0.96-1.75) | .093 |
| Smoking | 1.04 (0.87-1.23) | .671 |
| Hypertension | 1.35 (1.03-1.76) | .031 |
| History of previous infarction | 2.42 (1.40-4.20) | .002 |
| History of previous PTCA | 0.42 (0.19-0.92) | .030 |
| Extensive experience in use of radial access | 0.58 (0.42-0.82) | .002 |
| Ejection fraction at discharge | 0.99 (0.99-1.00) | .313 |
95CI%, 95% confidence interval; HR, hazard ratio; PTCA, percutaneous transluminal coronary angioplasty.
Bleeding is a relatively common complication of coronary angioplasty that is associated with an increased mortality.5,6 The present study shows that a radial approach in patients with STEMI decreases the risk of minor bleeding and improves the primary end point. Thus, a radial access route can improve the safety and prognosis of patients with STEMI who undergo emergency angioplasty. The radial approach facilitates arterial hemostasis, early diagnosis, and treatment of complications, and also permits earlier patient mobility.
There is a wide interhospital and geographical variability in the use of the radial approach. In the ACUITY trial,12 which recruited 13 819 patients with acute coronary syndrome from 600 centers in 10 countries, radial access was used in only 6.2% of the patients.12 In another recent 29-country study, which included 9126 high-risk patients with acute coronary syndrome treated with angioplasty, the radial (and brachial) approach was used in 13.5% of patients.13 In the United States, the CathPCI Registry of the National Cardiovascular Data Registry reported that in 1 110 150 patients between 2010 and 2011 the radial approach was used in 8.3% of diagnostic procedures and in only 6.9% of angioplasties; 13% of the hospitals never used it.1,14 In contrast, use of the radial approach in other centers can reach 88% in patients with STEMI.15 In the Examination study, radial access was used in 45% of patients. This wide variability in the choice of access is because the femoral approach is considered by some centers to be the standard or traditional technique, due to the “optimal” control of catheters and because it allows immediate access for larger diameter devices. These factors probably at least partly explain why, paradoxically, the femoral approach is used more frequently in high-risk patients.
In the current study, each operator and each center determined the choice of access. A femoral access route was more commonly used in women, diabetic patients, and those in worse clinical condition on admission. In this subgroup of patients, the femoral approach was possibly chosen due to a need to obtain a rapid vascular access in patients with poor clinical status and due to a weak radial pulse or a small radial artery.16,17 The experience of the operators in performing primary percutaneous transluminal coronary angioplasty by radial access would also affect the analysis of the composite end point.
In high-risk patients treated with angioplasty, anticoagulant and antiplatelet therapy, particularly with glycoprotein IIb/IIIa inhibitors, reduces the rate of ischemic complications, but at the expense of an increase in the incidence of bleeding complications.18 In these patients, the radial approach reduces the rates of major and minor bleeding and transfusions.19–21 In the present study, a radial access route was primarily used in patients previously treated with heparin and glycoprotein IIb/IIIa inhibitors. In these patients, the radial approach was probably chosen by the catheterization cardiologist to minimize the risk of bleeding, which could explain why no differences in the incidence of major bleeding or in the reduction of major cardiac events were associated with the vascular access.7 In a multicenter study that included 9126 patients, the radial access route was used in 13.5% of the patients, and no association was found between a radial approach and a reduction in major bleeding and mortality.13 In a metaanalysis of 3224 patients, the rates of major cardiac events were also similar between radial and femoral groups (2.5% vs 2.4%; P=.7).7 However, in agreement with other authors,7,22–25 the rate of minor bleeding complications was higher with femoral than radial access in the present study: 4.6% for femoral access vs 1.9% for radial access. In contrast, in the ACUITY trial,12 the incidence of minor bleeding was 7.4% and 7.2%, respectively, and in the RIFLES-STEACS trial,23 the incidence was 7.2% and 4.0%, respectively. These differences can be attributed to predominant use of the femoral approach in the ACUITY trial, whereas more extensive use was made of glycoprotein IIb/IIIa inhibitors in high-risk patients in the RIFLE-STEACS trial.12,25
In our study, the primary composite end point occurred less often in patients with STEMI who were operated on with a radial approach than with a femoral approach. These results are in accordance with those of the recent randomized study RIFLE-STEACS,23 which compared the radial and femoral approaches in patients with STEMI. The composite end point of cardiac death, stroke, myocardial infarction, revascularization of the target vessel, and bleeding occurred in 13.6% of the patients with radial access and in 21% of the patients with femoral access (P=.003). In another randomized study, which included patients with STEMI and patients with non—ST-segment elevation myocardial infarction, the radial approach decreased the incidence of the composite end point of death, myocardial infarction, stroke, and non-bypass surgery-related major bleeding in patients with STEMI (3.1% vs 5.2%; P=.026).24 In contrast, no differences were seen among patients with non—ST-segment elevation acute myocardial infarction, which underlines the particular importance of using a radial approach in patients with STEMI.22
LimitationsNo quantification of the catheterization cardiologist's level of experience with the radial approach was performed in the multicenter and multinational Examination study. However, the low incidence of bleeding reflects operator proficiency with both femoral and radial approaches. The effectiveness of the radial approach and the clinical results obtained are closely linked to the volume of procedures performed by this route.22
The Examination study was designed to compare the long-term clinical results of a drug-eluting stent with those of a conventional stent in consecutive patients. The type of vascular approach was chosen by the catheterization cardiologist. Accordingly, there may be a selection bias because the operator may have chosen the approach based on the clinical and/or anatomical conditions of the patient. However, given that the Examination trial included about 70% of the patients evaluated in the participating centers, the current study presents results that closely approximate the “real world” situation of emergency angioplasty in consecutive patients with STEMI, with operator experience and patient characteristics determining the choice of access route.
CONCLUSIONSIn patients with STEMI who undergo emergency angioplasty, a radial access route significantly reduces the incidence of minor bleeding complications compared with the femoral approach, which translates into a reduction in the composite end point, but without differences in the remaining components (all-cause death, myocardial infarction, revascularization of the target vessel). Our results indicate that, within the therapeutic strategy of patients with STEMI, the radial approach is an effective technique for improving prognosis and reducing bleeding complications.
CONFLICTS OF INTERESTSNone declared.