Extracorporeal membrane oxygenators (ECMO) are an efficient means of providing emergency pulmonary and circulatory support for patients with cardiogenic shock refractory to conventional intensive therapy or hypoxic-hypercapnic respiratory failure refractory to advanced ventilation strategies.1,2
Currently, ECMO use is limited to certain specialized tertiary centers that are both equipped to implement programs and experienced in their management. Hospitals that cannot undertake heart surgery or initiate ECMO find it extremely difficult to treat patients in refractory cardiogenic shock. Transferring patients to centers with better technical resources can be the only alternative but is often considered inviable due to their hemodynamic instability. Recent experience confirms that creating mobile heart surgery units—where support devices can be implanted in situ, followed by stabilization and transfer to a specialized center—offers these critical patients a chance of survival.3–5
The objective of the present study is to determine the feasibility and safety of an inter-hospital transfer program for critical patients with ECMO support. We describe the logistic problems, indications, complications, and clinical course of the patients enrolled.
The Cardio-Thoracic Surgery Service of the J.W. Goethe University Hospital, Frankfurt, Germany, established a Distance Cardiac Care Unit to provide ECMO circulatory support for institutions in the Hessen area lacking this therapeutic resource. The unit was created in December 2011 and consists of a perfusionist and a heart surgeon permanently on call and in direct contact with the secondary hospitals’ critical care units.
Veno-arterial ECMO support implantation was indicated in cases of cardiogenic shock refractory to conventional critical care treatment (systolic blood pressure under 80mmHg, administration of at least two inotropic agents, and/or counterpulsation balloon and signs of inadequate perfusion).1,2 Veno-venous support was indicated in cases of hypoxic-hypercapnic respiratory failure refractory to advanced ventilation strategies (respiratory distress syndrome).1,6
We used the PLS extracorporeal system (Maquet AG; Germany), consisting of a closed polyvinyl circuit, membrane oxygenator and centrifugal pump. The patient was intubated using percutaneous venous cannulas (17-25 Fr) and arterial cannulas (18-21 Fr). In patients with respiratory failure only, veno-venous ECMO support was established by using the Seldinger technique of inserting cannulas in both femoral veins, lodging one in the right atrium (outflow) and the other in the inferior vena cava (inflow). In cases of cardiogenic shock, veno-arterial ECMO support was surgically implanted in the deltopectoral groove giving access to the axillary artery to connect the arterial line and a venous cannula inserted percutaneously in the inferior vena cava. Procedures were performed under general anesthetic in the patient's intensive care unit bed, after administering 10 000 U heparin.
From December 2011 to April 2013, 10 patients in critical condition (8 men, 2 women), with mean age 46.7 (standard deviation, 15.3) (range, 18-61) years, required ECMO support in secondary hospitals in Hessen; inter-hospital transfer to our center took place post-implantation. In all cases, transfer was by road, in a mobile intensive care unit. The heart support team's preparation time was 25minutes and time from call-out to implantation, 90minutes.
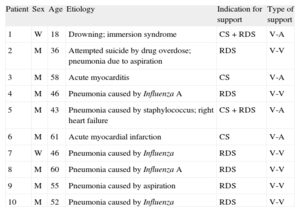
The demographic characteristics of patients and underlying causes of their need for cardiopulmonary support are in Table 1. Six patients presented with severe pulmonary failure only and received veno-venous ECMO implants; 2 in cardiopulmonary failure and 2 in cardiogenic shock had veno-arterial ECMO implantations. Once the indication for support and support type had been established, patients were connected to the ECMO circuit. There were no complications due to percutaneous venous cannulation. A surgical approach using axillary artery access was needed in 4 patients, with no complications.
Demographic Characteristics of Patients Enrolled. Causes of Cardiorespiratory Failure. Indications for ECMO and ECMO Support Type
| Patient | Sex | Age | Etiology | Indication for support | Type of support |
| 1 | W | 18 | Drowning; immersion syndrome | CS + RDS | V-A |
| 2 | M | 36 | Attempted suicide by drug overdose; pneumonia due to aspiration | RDS | V-V |
| 3 | M | 58 | Acute myocarditis | CS | V-A |
| 4 | M | 46 | Pneumonia caused by Influenza A | RDS | V-V |
| 5 | M | 43 | Pneumonia caused by staphylococcus; right heart failure | CS + RDS | V-A |
| 6 | M | 61 | Acute myocardial infarction | CS | V-A |
| 7 | W | 46 | Pneumonia caused by Influenza | RDS | V-V |
| 8 | M | 60 | Pneumonia caused by Influenza A | RDS | V-V |
| 9 | M | 55 | Pneumonia caused by aspiration | RDS | V-V |
| 10 | M | 52 | Pneumonia caused by Influenza | RDS | V-V |
CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenator; M, man; RDS, respiratory distress syndrome; V-A, veno-arterial ECMO; V-V, veno-venous ECMO; W, woman.
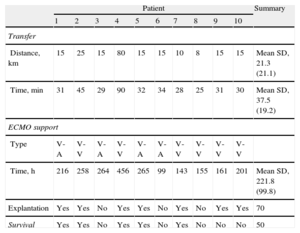
Table 2 shows the mean transfer distance was 21 (8-80) km and unit-to-unit transport time, 37 (25-90) min. No device-related logistical or technical complications arose during implantation or transfer. None of the patients had transfer-related complications, morbidity, or mortality.
Inter-hospital Distances and Transport Times for Critical Patients With ECMO Support. Type of ECMO Support Implanted and Support Duration. Patient Postoperative Clinical Course
| Patient | Summary | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Transfer | |||||||||||
| Distance, km | 15 | 25 | 15 | 80 | 15 | 15 | 10 | 8 | 15 | 15 | Mean SD, 21.3 (21.1) |
| Time, min | 31 | 45 | 29 | 90 | 32 | 34 | 28 | 25 | 31 | 30 | Mean SD, 37.5 (19.2) |
| ECMO support | |||||||||||
| Type | V-A | V-V | V-A | V-V | V-A | V-A | V-V | V-V | V-V | V-V | |
| Time, h | 216 | 258 | 264 | 456 | 265 | 99 | 143 | 155 | 161 | 201 | Mean SD, 221.8 (99.8) |
| Explantation | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes | 70 |
| Survival | Yes | Yes | No | Yes | Yes | No | Yes | No | No | No | 50 |
ECMO, extracorporeal membrane oxygenator; SD, standard deviation; V-A, veno-arterial ECMO; V-V, veno-venous ECMO.
Data are expressed as % or mean (standard deviation).
Table 2 shows the patients’ postoperative clinical course. The ECMO systems were explanted after a mean 9.7 (4-19) days on support. Explantation was successful in 70% of patients (7/10) and survival to discharge was 50%. In-hospital mortality was similar to that of 119 patients receiving ECMO support in our center during the same period (51%).
We conclude that, in our experience, emergency implantation of ECMO-type cardiopulmonary support in external institutions and subsequent transfers of patients to regional referral centers are feasible in practice, with reasonable mid-term results.