The incidence of acute coronary syndromes is high in the elderly population. Bleeding is associated with a poorer prognosis in this clinical setting. The available bleeding risk scores have not been validated specifically in the elderly. Our aim was to assess predictive ability of the most important bleeding risk scores in patients with acute coronary syndrome aged ≥ 75 years.
MethodsWe prospectively included consecutive acute coronary syndromes patients. Baseline characteristics, laboratory findings, and hemodynamic data were collected. In-hospital bleeding was defined according to CRUSADE, Mehran, ACTION, and BARC definitions. CRUSADE, Mehran, and ACTION bleeding risk scores were calculated for each patient. The ability of these scores to predict major bleeding was assessed by binary logistic regression, receiver operating characteristic curves, and area under the curves.
ResultsWe included 2036 patients, with mean age of 62.1 years; 369 patients (18.1%) were ≥ 75 years. Older patients had higher bleeding risk (CRUSADE, 42 vs 22; Mehran, 25 vs 15; ACTION, 36 vs 28; P<.001) and a slightly higher incidence of major bleeding events (CRUSADE bleeding, 5.1% vs 3.8%; P=.250). The predictive ability of these 3 scores was lower in the elderly (area under the curve, CRUSADE: 0.63 in older patients, 0.81 in young patients; P=.027; Mehran: 0.67 in older patients, 0.73 in younger patients; P=.340; ACTION: 0.58 in older patients, 0.75 in younger patients; P=.041).
ConclusionsCurrent bleeding risk scores showed poorer predictive performance in elderly patients with acute coronary syndromes than in younger patients.
Keywords
Bleeding complications are associated with a less favorable prognosis in patients with an acute coronary syndrome (ACS).1–3 Clinical practice guidelines4,5 recommend basing therapy strategies for these patients on the risk of both ischemic and bleeding events. Hence, over the last few years, various risk scores have been designed to predict bleeding complications in this scenario.6–8
The incidence of acute myocardial infarction increases with age and is particularly elevated in the elderly population.9 Comorbid conditions and frailty are common in older acute myocardial infarction patients and are associated with a higher incidence of complications, including bleeding complications, and with expenditure of health resources.10 For this reason, health care for acute myocardial infarction in the elderly may become an important public health problem in the coming years. Nonetheless, patients of advanced age are usually underrepresented in clinical trials,11,12 and clinical evidence on the management and risk stratification in elderly patients with an ACS is scarce. There is no information on the performance of bleeding risk scores in this age group.
Therefore, the aims of this study were: a) analyze the incidence of bleeding complications and their characteristics in patients ≥ 75 years, and b) to evaluate the predictive ability of the main bleeding risk scores in this age group compared with that in the remaining patients in a consecutive cohort of ACS patients admitted to the coronary unit of a third-level hospital.
METHODSStudy DesignThis is a prospective, observational study performed in a single referral hospital for ACS. We prospectively included all ACS patients admitted to the coronary unit of our center between October 2009 and June 2012. The diagnosis and therapeutic management of the patients was carried out in accordance with current recommendations.4,5
Definitions and Data CollectionTrained staff prospectively compiled the study data using a standardized questionnaire. They recorded the patients’ baseline characteristics, clinical history, biochemical and electrocardiographic findings, echocardiographic and angiographic parameters, procedures carried out, treatment administered during hospitalization, and in-hospital complications and deaths. The incidence and site of the in-hospital bleeding event was recorded, as well as the need for transfusion of blood products, the hemodynamic repercussions, and the intervention requirements.
Bleeding EventsThe CRUSADE,6 Mehran,7 ACTION,8 and BARC13 definitions were used to assign the bleeding events. All the elements comprising these definitions of bleeding were included in the data collection form. For each patient, the CRUSADE,6 Mehran,7 and ACTION8 bleeding risk scores were calculated, as well as the GRACE14 risk score. In the analysis of the CRUSADE, Mehran and ACTION scores by risk group, the previously defined categories6–8 for each of the scores were used.
The hemodynamic parameters (heart rate, systolic arterial pressure) and Killip grade were recorded at admission to the coronary unit. Creatinine clearance was calculated using the Cockcroft-Gault formula.15
Quantification of coronary disease was performed attending to the number of coronary arterial territories (left anterior descending, circumflex, right coronary) with stenosis of the arterial lumen ≥ 70% (≥ 50% in the case of the left common trunk). The degree of stenosis was quantified by visual analysis.
Statistical AnalysisData analysis was performed using the PASW Statistics 18 statistical package (Chicago, Illinois, United States) and the R 3.0.1 software. Categorical values are expressed as number and percentage, and quantitative variables as mean (standard deviation). Variables with a nonnormal distribution are expressed as the median [interquartile range]. The Kolmogorov-Smirnoff test was used to analyze the normality of the distributions.
Comparisons between categorical variables were carried out with the chi-square test or Fisher's exact test, when appropriate. To analyze the incidence of bleeding events according to the various risk categories, the chi-square test was again used, together with the Mantel and Haenszel test for linear trends. Comparisons between quantitative variables were performed with the Student t test.
Binary logistic regression analysis was used to determine the predictive ability of the various bleeding risk scores, with calculation of the ROC (receiver operating characteristic) curves and the corresponding AUC (area under the ROC curve). Comparisons between AUCs were made with the nonparametric method of DeLong.16 Two types of comparisons were performed: First, in patients ≥ 75 years, the ability of the CRUSADE, Mehran, and ACTION scores to predict bleeding according to the definitions used to design each score, was compared with the performance of the scores in younger patients. The comparison was made using the DeLong method with independent samples. Second, the capacity of the CRUSADE, Mehran, and ACTION bleeding risk scores and the GRACE score to predict major bleeding events was compared according to the recent BARC13 bleeding definitions. For reasons of clinical relevance, BARC types 3 and 5 were considered for this purpose. This comparison was performed in both the group aged ≥ 75 years and in younger patients, using the DeLong method with paired samples. Patients with missing risk score data were excluded from the analysis.
RESULTSThe study included 2036 patients (mean age, 62.1 years), 1570 (77.1%) of whom were men; 369 patients (18.1%) were aged ≥ 75 years. The characteristics of the study population according to age are shown in Table 1.
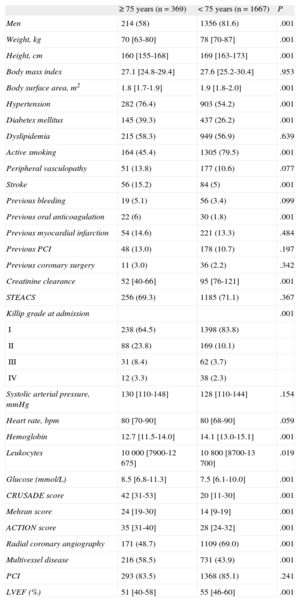
Clinical Characteristics According to Age
| ≥ 75 years (n=369) | < 75 years (n=1667) | P | |
| Men | 214 (58) | 1356 (81.6) | .001 |
| Weight, kg | 70 [63-80] | 78 [70-87] | .001 |
| Height, cm | 160 [155-168] | 169 [163-173] | .001 |
| Body mass index | 27.1 [24.8-29.4] | 27.6 [25.2-30.4] | .953 |
| Body surface area, m2 | 1.8 [1.7-1.9] | 1.9 [1.8-2.0] | .001 |
| Hypertension | 282 (76.4) | 903 (54.2) | .001 |
| Diabetes mellitus | 145 (39.3) | 437 (26.2) | .001 |
| Dyslipidemia | 215 (58.3) | 949 (56.9) | .639 |
| Active smoking | 164 (45.4) | 1305 (79.5) | .001 |
| Peripheral vasculopathy | 51 (13.8) | 177 (10.6) | .077 |
| Stroke | 56 (15.2) | 84 (5) | .001 |
| Previous bleeding | 19 (5.1) | 56 (3.4) | .099 |
| Previous oral anticoagulation | 22 (6) | 30 (1.8) | .001 |
| Previous myocardial infarction | 54 (14.6) | 221 (13.3) | .484 |
| Previous PCI | 48 (13.0) | 178 (10.7) | .197 |
| Previous coronary surgery | 11 (3.0) | 36 (2.2) | .342 |
| Creatinine clearance | 52 [40-66] | 95 [76-121] | .001 |
| STEACS | 256 (69.3) | 1185 (71.1) | .367 |
| Killip grade at admission | .001 | ||
| I | 238 (64.5) | 1398 (83.8) | |
| II | 88 (23.8) | 169 (10.1) | |
| III | 31 (8.4) | 62 (3.7) | |
| IV | 12 (3.3) | 38 (2.3) | |
| Systolic arterial pressure, mmHg | 130 [110-148] | 128 [110-144] | .154 |
| Heart rate, bpm | 80 [70-90] | 80 [68-90] | .059 |
| Hemoglobin | 12.7 [11.5-14.0] | 14.1 [13.0-15.1] | .001 |
| Leukocytes | 10 000 [7900-12 675] | 10 800 [8700-13 700] | .019 |
| Glucose (mmol/L) | 8.5 [6.8-11.3] | 7.5 [6.1-10.0] | .001 |
| CRUSADE score | 42 [31-53] | 20 [11-30] | .001 |
| Mehran score | 24 [19-30] | 14 [9-19] | .001 |
| ACTION score | 35 [31-40] | 28 [24-32] | .001 |
| Radial coronary angiography | 171 (48.7) | 1109 (69.0) | .001 |
| Multivessel disease | 216 (58.5) | 731 (43.9) | .001 |
| PCI | 293 (83.5) | 1368 (85.1) | .241 |
| LVEF (%) | 51 [40-58] | 55 [46-60] | .001 |
LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome.
Multivessel disease: significant stenosis (> 60%) in 2 or more coronary artery territories (left anterior descending, right coronary, circumflex).
Categorical variables are expressed as the No. (%) and quantitative variables with nonnormal distribution as the median [interquartile range].
In general, elderly patients had a larger number of cardiovascular risk factors and other comorbidities, a greater incidence of signs of heart failure, and significantly lower glomerular filtration and hemoglobin values at admission than the remaining patients. In addition, coronary artery disease was more diffuse and left ventricular function was poorer in the older group. Furthermore, the overall bleeding risk was significantly higher in this group, as reflected by higher scores on each of the 3 bleeding risk scales compared with younger patients. The number of patients with missing data was 162 of 2036 (8%) for the CRUSADE score, 252 of 2036 (12.4%) for Mehran, and 85 of 2036 (4.2%) for ACTION. There were no significant differences between the age groups in the percentage of patients with missing values.
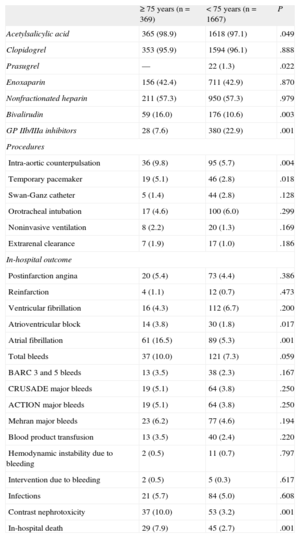
Data regarding in-hospital management and outcome by age groups is shown in Table 2. Coronary angiography by radial access was used less often, and treatment with prasugrel or glycoprotein IIb/IIIa platelet inhibitors was less commonly prescribed in older patients. In contrast, bivalirudin treatment was used more often in this group. As to invasive procedures, there was greater use of intra-aortic counterpulsation and temporary pacemakers in elderly patients.
In-hospital Management and Outcome According to Age
| ≥ 75 years (n=369) | < 75 years (n=1667) | P | |
| Acetylsalicylic acid | 365 (98.9) | 1618 (97.1) | .049 |
| Clopidogrel | 353 (95.9) | 1594 (96.1) | .888 |
| Prasugrel | — | 22 (1.3) | .022 |
| Enoxaparin | 156 (42.4) | 711 (42.9) | .870 |
| Nonfractionated heparin | 211 (57.3) | 950 (57.3) | .979 |
| Bivalirudin | 59 (16.0) | 176 (10.6) | .003 |
| GP IIb/IIIa inhibitors | 28 (7.6) | 380 (22.9) | .001 |
| Procedures | |||
| Intra-aortic counterpulsation | 36 (9.8) | 95 (5.7) | .004 |
| Temporary pacemaker | 19 (5.1) | 46 (2.8) | .018 |
| Swan-Ganz catheter | 5 (1.4) | 44 (2.8) | .128 |
| Orotracheal intubation | 17 (4.6) | 100 (6.0) | .299 |
| Noninvasive ventilation | 8 (2.2) | 20 (1.3) | .169 |
| Extrarenal clearance | 7 (1.9) | 17 (1.0) | .186 |
| In-hospital outcome | |||
| Postinfarction angina | 20 (5.4) | 73 (4.4) | .386 |
| Reinfarction | 4 (1.1) | 12 (0.7) | .473 |
| Ventricular fibrillation | 16 (4.3) | 112 (6.7) | .200 |
| Atrioventricular block | 14 (3.8) | 30 (1.8) | .017 |
| Atrial fibrillation | 61 (16.5) | 89 (5.3) | .001 |
| Total bleeds | 37 (10.0) | 121 (7.3) | .059 |
| BARC 3 and 5 bleeds | 13 (3.5) | 38 (2.3) | .167 |
| CRUSADE major bleeds | 19 (5.1) | 64 (3.8) | .250 |
| ACTION major bleeds | 19 (5.1) | 64 (3.8) | .250 |
| Mehran major bleeds | 23 (6.2) | 77 (4.6) | .194 |
| Blood product transfusion | 13 (3.5) | 40 (2.4) | .220 |
| Hemodynamic instability due to bleeding | 2 (0.5) | 11 (0.7) | .797 |
| Intervention due to bleeding | 2 (0.5) | 5 (0.3) | .617 |
| Infections | 21 (5.7) | 84 (5.0) | .608 |
| Contrast nephrotoxicity | 37 (10.0) | 53 (3.2) | .001 |
| In-hospital death | 29 (7.9) | 45 (2.7) | .001 |
GP IIb/IIIa, glycoprotein IIb/IIIa platelet inhibitors.
Categorical values are expressed as No. (%).
The in-hospital outcome also showed some differences between the age groups. There was a higher incidence of atrioventricular block, atrial fibrillation, and angiographic contrast-related nephrotoxicity in elderly patients, as well as greater in-hospital mortality. The incidence of bleeding complications was slightly higher in the older group, although this difference did not reach statistical significance for any of the definitions used. Nor were there differences in the transfusion requirements, development of hemodynamic instability due to bleeding, or the need for surgery to resolve bleeding.
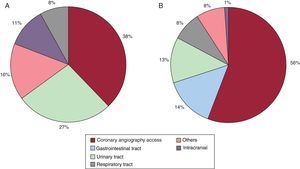
The analysis of the location of bleeding complications according to age showed some relevant differences. In the elderly, the most common sites were the femoral access, urinary tract, and an intracranial location. In contrast, in younger patients, the most common sites were the access route, digestive tract, and urinary tract. The bleeding sites in both age groups are shown in Figure 1.
In patients < 75 years, the incidence of in-hospital bleeding was significantly associated with the various risk categories of each of the scores analyzed, showing an increase in parallel to each risk category. Thus, the incidence of CRUSADE major bleeding events according to the CRUSADE quintiles was as follows: very low risk, 7 of 790 (0.9%); low risk, 10 of 389 (2.6%); intermediate risk, 12 of 177 (6.8%); high risk, 9 of 100 (9%); and very high risk, 19 of 84 (22.6%) (P<.001; test for linear trend, P<.001).
The incidence of ACTION major bleeding events in each ACTION category was the following: very low risk, 2 of 166 (1.2%); low risk, 17 of 877 (1.9%); intermediate risk, 25 of 480 (5.2%); high risk, 10 of 60 (16.7%), and very high risk, 6 of 17 (35.3%) (P<.001; test for linear trend, P<.001).
Finally, the incidence of Mehran major bleeding events according to the Mehran categories was: low risk, 4 of 361 (1.1%); intermediate risk, 7 of 414 (1.7%); high risk, 21 of 309 (6.8%), and very high risk, 35 of 368 (9.5%) (P<.001; test for linear trend, P<.001).
In contrast, in elderly patients, the association between in-hospital bleeding and the various risk categories of the scores analyzed did not reach statistical significance. In this patient population the incidence of CRUSADE major bleeding events according to the CRUSADE quintiles was: very low risk, 1 of 20 (5%); low risk, 1 of 62 (1.6%); intermediate risk, 3 of 73 (4.1%); high risk, 4 of 83 (8.3%); very high risk, 8 of 96 (8.3%) (P=.434; test for linear trend, P=.102).
The incidence of ACTION major bleeding events in each of the ACTION categories was: very low risk, 0 of 1 (0%); low risk, 2 of 80 (2.5%); intermediate risk, 11 of 202 (5.4%); high risk, 3 of 58 (5.2%), and very high risk, 1 of 10 (10%) (P=.781; test for linear trend, P=.271).
Finally, the incidence of Mehran major bleeding events according to the Mehran categories was: intermediate risk, 0 of 29 (0%); high risk, 3 of 56 (5.4%), and very high risk, 19 of 247 (7.7%) (P=.265; test for linear trend, P=.113).
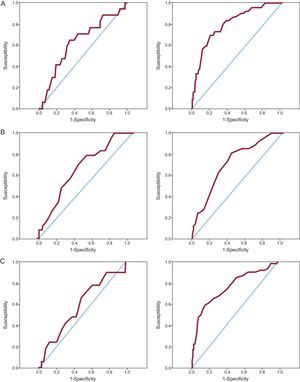
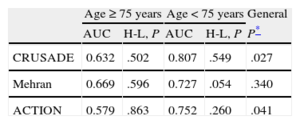
Predictive Capacity According to AgeThe 3 bleeding risk scores showed poorer predictive performance in elderly patients than in younger ones. The differences seen when using the CRUSADE and ACTION scores were clearly significant. The predictive capacity of the Mehran score in the elderly group was also lower than that obtained in younger patients, but this difference did not reach statistical significance (Table 3). The ROC curves of the CRUSADE, Mehran, and ACTION scores for predicting bleeding complications in elderly and younger patients are shown in Figure 2. Calibration, expressed by the Hosmer-Lemeshow test, was adequate in most cases, although it was somewhat inferior for the Mehran score in the younger group.
Ability of the CRUSADE, Mehran, and ACTION Scores to Predict Major Bleeding (According to the Definitions for Each Score) by Age
| Age ≥ 75 years | Age < 75 years | General | |||
| AUC | H-L, P | AUC | H-L, P | P* | |
| CRUSADE | 0.632 | .502 | 0.807 | .549 | .027 |
| Mehran | 0.669 | .596 | 0.727 | .054 | .340 |
| ACTION | 0.579 | .863 | 0.752 | .260 | .041 |
AUC, area under the receiver operating characteristic curve; H-L, Hosmer-Lemeshow test.
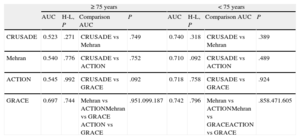
None of the 3 bleeding risk scores showed a predictive capacity significantly superior to the other 2 scores in either the elderly group or in younger patients (Table 4).
Comparative Analysis of the Ability of the CRUSADE, Mehran, and ACTION Scores to Predict Major Bleeding (BARC 3 and 5 Bleeds) in the 2 Age Groups
| ≥ 75 years | < 75 years | |||||||
| AUC | H-L, P | Comparison AUC | P | AUC | H-L, P | Comparison AUC | P | |
| CRUSADE | 0.523 | .271 | CRUSADE vs Mehran | .749 | 0.740 | .318 | CRUSADE vs Mehran | .389 |
| Mehran | 0.540 | .776 | CRUSADE vs ACTION | .752 | 0.710 | .092 | CRUSADE vs ACTION | .489 |
| ACTION | 0.545 | .992 | CRUSADE vs GRACE | .092 | 0.718 | .758 | CRUSADE vs GRACE | .924 |
| GRACE | 0.697 | .744 | Mehran vs ACTIONMehran vs GRACE ACTION vs GRACE | .951.099.187 | 0.742 | .796 | Mehran vs ACTIONMehran vs GRACEACTION vs GRACE | .858.471.605 |
AUC, area under the receiver operating characteristic curve; H-L, Hosmer-Lemeshow test.
The performance of the GRACE score by age was slightly better than that of the 3 bleeding risk scores (Table 4). In younger patients, GRACE demonstrated adequate predictive ability, with no significant differences relative to the 3 bleeding risk scores. In contrast, the predictive ability of GRACE in the elderly group (even though it was lower than that seen in younger patients) experienced a smaller loss than that observed with the other scores. Therefore, even though there were no statistically significant differences, there was a trend to a higher AUC value for the GRACE score than for the remaining scores in elderly patients.
DISCUSSIONTwo main findings were obtained from this study: a) the incidence of bleeding complications in patients ≥ 75 years was slightly higher than that of younger patients, with significant differences in the site of bleeding, but no differences in bleeding severity, and b) the main instruments currently available for bleeding risk stratification clearly show poorer performance in patients of more advanced age.
The evident prognostic implications of bleeding complications in ACS patients1–3 confers particular importance to bleeding risk stratification in this context. Although stratification of bleeding risk is less extensively developed than assessment of the risk of recurrent ischemic events,17,18 a number of tools designed to predict bleeding complications in this scenario are available.6–8 Validation of these risk scores in our setting is particularly important because of the notable demographic, ethnic, and sociodemographic differences of our population relative to the populations that were used as a basis in their development. In this regard, Abu-Assi et al19 analyzed a Spanish cohort of 782 consecutive patients with non–ST-segment elevation ACS and found that the CRUSADE score showed optimal discriminatory ability in this context. In another interesting contribution, Abu-Assi et al20 compared the predictive ability of the CRUSADE, Mehran and ACTION scores in a large series of 4500 consecutive ACS patients and reported that the predictive ability of the 3 scores was adequate in this scenario (C statistic > 0.70, except in patients managed conservatively), although it was somewhat superior with CRUSADE and ACTION. Therefore, the predictive ability of the main bleeding risk scores can be considered adequately valid in our setting.
It is evident, however, that risk stratification in elderly ACS patients presents certain peculiarities.21–23 Comorbidities and/or frailty are common in this population, and these factors can be associated with a higher incidence of complications and adverse effects related to the drugs most often used for treating this condition. Furthermore, despite the growing incidence of ACS in elderly patients,9 this population is poorly represented in the related studies;11,12 hence, specific information on their management and risk stratification is scarce. To our knowledge, there are no studies analyzing the performance of bleeding risk scores specifically in elderly patients. Although it was not one of the principle aims of their study, Abu-Assi et al19 reported adequate predictive ability of the CRUSADE score in patients ≥ 75 years (n=257; C statistic=0.80; 95% confidence interval, 0.75-0.85). Certain differences between the population included in that study and the patients in our series might explain the divergences with our findings. Patients in the Abu-Assi study had a higher overall bleeding risk than those in our series (CRUSADE median 30 vs 23, respectively). Moreover, the fact that their study population only included cases of non–ST-segment elevation ACS could imply that their patients received more intensive and prolonged antithrombotic treatment, which, in turn, could account for the greater incidence of major bleeding events recorded (9.5%). There are no references to the performance of the Mehran and ACTION scores in elderly patients.
Our data in the younger population show poorer calibration of the Mehran score in this age group. The Mehran instrument was created based on patients in clinical trials (ACUITY, HORIZONS), whereas CRUSADE and ACTION used populations from registries, with all the differences in clinical characteristics and comorbidities that this might imply. Furthermore, the Mehran score is the only score of the 3 scores studied that does not incorporate variables such as the Killip class or hemodynamic data (heart rate or blood pressure), and in contrast to the others, it does include variables related to pharmacological treatment. Although other authors have not found these calibration differences in our setting,20 the differential characteristics of the Mehran score could partly explain the differences in calibration seen in our series, although this finding would have to be corroborated in larger patient samples.
Apart from these findings, the data from our series are the first to show a consistently poorer discriminative ability of the 3 bleeding risk scores in ACS patients of advanced age. In our opinion, the commonly coexisting comorbidities and frailty of these patients can notably affect their management and clinical evolution, and imply that predicting their risk of ischemic and hemorrhagic events is a much more complex task. This type of variable is rarely measured in trials and registries of cardiovascular disease, and the studies from which the CRUSADE, Mehran, and ACTION scores are derived are not an exception in this regard. Furthermore, elderly patients were notably underrepresented in the study populations,6–8 in which the mean age was between 62 and 67 years. We believe that this is one of the main reasons for the weaker predictive ability documented in elderly patients in our series. The lower capacity of the GRACE score to predict bleeding in the elderly population in our series (although somewhat less evident compared with that of the bleeding risk scores) may be due to similar reasons.
Assessment of variables such as frailty, comorbidities, and functional status could provide relevant information and markedly contribute to improving prognostic stratification in elderly patients with ACS.24
In our study, the incidence of bleeding events was higher in the elderly group than in the remaining patients, although perhaps to a lesser degree than would be expected. Age is a recognized predictor of bleeding complications in ACS patients.25,26 In fact, it is a part of the ACTION8 and Mehran7 scores and indirectly, through the glomerular filtration rate, of the CRUSADE score.6 The more conservative antithrombotic therapeutic management of the elderly in our series (lower use of potent antiplatelet agents, such as prasugrel and glycoprotein IIb/IIIa platelet inhibitors, and greater use of theoretically safer agents27 such as bivalirudin) may have contributed to yielding smaller differences in the bleeding events according to age.
The distribution of the sites of bleeding complications provided interesting results. Despite the greater use of the femoral access in elderly patients (probably influenced by the anatomical characteristics of the brachial circulation in these patients and their more unfavorable hemodynamic status at admission) there was a smaller percentage of access-related bleeding in this group. Again, we believe that the more conservative pharmacological management in patients of advanced age may be implicated in this apparent paradox, as well as in the lower presence of gastrointestinal bleeding seen in these patients. Furthermore, the results highlight the greater role of urinary tract bleeding in the elderly, likely conditioned by a greater burden of preexisting comorbidity, and of intracranial bleeding, an association that has been extensively described.
LimitationsOur research has the limitations inherent to a single center study, in which the therapeutic management was fairly homogeneous and there was an elevated presence of ST-segment elevation ACS treated by primary angioplasty. Furthermore, inclusion of patients admitted to the coronary unit only may have implied some degree of bias. Therefore, until our findings can be confirmed in other scenarios, they should only be considered applicable to populations with a similar profile undergoing comparable clinical management. The sample size, particularly in the elderly group, is another limitation of the study. However, although a low number of events can limit the statistical precision of ROC curve calculation, we believe that the notables differences seen (which, moreover, concurred in the 3 bleeding risk scores studied), are not attributable to a small sample size, particularly taking into account the conservative effect of the statistical approach used.28 Another factor that should be mentioned is that the Mehran score was designed to predict bleeding up to 30 days and not only during the period of hospitalization, as in our study. Finally, recording of patient frailty and associated comorbidities might have provided relevant information for the association studied.
Despite these limitations, we believe that the findings from our study clearly illustrate the shortcomings of current bleeding risk stratification in the elderly ACS population, and the need to develop new, more precise tools for this purpose.
CONCLUSIONESElderly patients in our series had a higher risk of bleeding and a slightly higher incidence of bleeding complications than younger patients. This study is the first to report suboptimal performance of the main bleeding risk scores in elderly patients with ACS. Assessment of factors such as patient frailty and comorbidity could, in our opinion, contribute to improving the predictive ability of these tools in this clinical setting.
CONFLICTS OF INTERESTSNone declared.