Implantable loop recorders have proven efficacy in the study of patients with syncope and palpitations. Remote monitoring of patients with pacemakers and implantable cardioverter-defibrillators has been shown to be safe and effective. The purpose of this study was to analyze the safety and effectiveness of remote monitoring in patients with an implantable loop recorder.
MethodsRetrospective observational study in which 109 patients with an implantable loop recorder were analyzed and 2 population groups were compared: 1 receiving conventional follow-up consisting of 3-monthly office visits (41 patients) and 1 with remote monitoring via monthly telephone transmissions and yearly visits (68 patients). The mean follow-up was 64 weeks (range, 0.57-164.57 weeks). The study analyzed diagnosis of a significant event, defined as any event that led to a therapeutic approach and explained the symptoms leading to the implant, as well as the mean time from implant to diagnosis and the specific treatment.
ResultsA significant event was diagnosed in 82.6% of patients; of these, 54.4% had a normal electrocardiogram; 26.7%, asystole; 15.6%, tachycardia, and 3.3%, bradycardia. The mean time from implant to diagnosis was 260 days (range, 5-947 days) in conventional follow-up, compared with 56 days (range, 0-650 days) in patients with remote monitoring (P<.01), which led to targeted treatment in this group 187 days earlier, on average, with no secondary complications.
ConclusionsRemote monitoring of patients with an implantable loop recorder can significantly shorten the time to diagnosis and targeted treatment, without adversely affecting patient safety.
Keywords
Syncope continues to be one of the most common disorders among the general population; approximately 15% to 30% have had 1 syncopal episode in their lifetime, and among all patients who attend the emergency room in Europe the incidence is 1% (range, 0.9%-1.7%).1,2 In recent years, considerable advances have been made in understanding and diagnosing this condition, and the number of patients with a final diagnosis of syncope of undetermined origin is increasingly smaller.3–5 This reduction has been greatly enhanced by the development and widespread use of the implantable loop recorder (ILR).1 The ILR is a subcutaneous device for continuous electrocardiographic monitoring of patients that can be enormously useful in the diagnosis of syncope of undetermined origin after the initial assessment and in patients with unrecorded palpitations that occur occasionally, which has led to a significant rise in the number of implants of this device in recent years.6 Patients with an ILR have traditionally been followed by outpatient clinics, with frequent visits to avoid the loss of information due to device memory overflow. The technology required for remote monitoring (RM) has become available in recent years. From home, the patient performs regular telephone transmissions of stored records, which are then reviewed by the hospital staff who, if necessary, contact the patient. This shortens the time needed to obtain relevant information and to take appropriate therapeutic action. The efficacy of the ILR as a diagnostic tool to investigate syncope has already been demonstrated in various studies in recent years, such as the pioneer study by Krahn et al.7 and other subsequent studies.8,9 However, although RM has already been proven to be effective in the follow-up of patients with a pacemaker (PM) or implantable cardioverter-defibrillator (ICD) in various studies,10–16 there is a paucity of data available on the ILR.17,18 The purpose of this study was to demonstrate the greater effectiveness of RM in patients with an ILR, without reducing health care safety and quality. We carried out a historical cohort study of all patients who received an ILR at our hospital between January 2003 and December 2010, comparing 2 population groups: patients participating in 3-monthly on-site conventional follow-up (CF) (ILR implant between March 2003 and October 2010) versus patients with RM (implanted between June 2009 and December 2010). The time cut-off was the initial use of RM in 2009 (all subsequent patients until database close-out versus all subjects since 2003), except for patients who did not agree to remote follow-up, from those who were followed up using the conventional approach.
METHODSPatient PopulationThe study included all patients who received an ILR (Medtronic Reveal® DX/XT) between January 2003 and December 2010 for investigation of syncope or palpitations of undetermined origin, regardless of whether the patient had structural heart disease or not. We also included patients with Brugada syndrome and nonspecific symptoms (presyncope, dizziness, etc.) but without syncope or palpitations who received an ILR.
Study DesignA historical cohort study was performed by comparing 2 models for the follow-up of patients with ILR: a conventional model that consisted of 3-monthly on-site follow-up in which the device was interrogated and the patient was clinically assessed, versus a follow-up model based on telephone transmissions of records stored in the ILR, routinely carried out by the patient at home every month or within 24 h of a significant event, and on-site visits scheduled 1 year after implantation. A significant event was considered to be electrocardiographic detection of asystole, bradycardia, tachycardia, or normal electrocardiogram or with slight rhythm variation (according to the ISSUE classification19 [Table 1]) related to the symptoms that led to ILR implantation and identified by the patient. In the RM model, a 2-way telephone contact communication system between the patient and the arrhythmia unit was implemented for cases of syncope or a significant event recorded in the transmissions.
Classification of Electrocardiographic Recordings Obtained With an Implantable Loop Recorder14
| Type 1 | Asystole>3 s |
| Type 2 | Bradycardia<40 bpm |
| Type 3 | Symptoms; absent or slight variation in rhythm |
| Type 4 | Tachycardia>120 bpm |
Adapted from the International Study on Syncope of Unknown Etiology classification.
The variables analyzed were the baseline characteristics of the sample (age, sex, history of structural heart disease, number of previous syncopal episodes, and indication of ILR); CF/RM; diagnosis of a significant event according to the ISSUE classification19; number of scheduled, unscheduled, and emergency room visits; number of electrocardiographic transmissions sent (total and for symptoms); mean times from symptom onset to ILR implantation, symtom onset to diagnosis of a significant event, symptom onset to start of treatment, ILR implantation to diagnosis of a significant event, and implantation to start of a specific treatment, as well as the type of treatment.
The ILR was subcutaneously implanted using the routine technique. The implant area is located between the first and fourth ribs, in the area defined by the left parasternal and midclavicular lines. The Reveal® DX/XT ILR device (Medtronic) has a mean longevity of 3 years and a total memory capacity of 49.5 min. This closed-loop recording system records the patient's electrocardiogram by using 2 electrodes at the rear of the device housing. Events can be recorded in 2 ways: automatic, when the device detects bradycardia, asystole, ventricular or atrial tachycardia, or atrial fibrillation, with programmable cut-off points, or patient-activated in the case of symptoms. Up to 27 automatically-activated and 3 patient-activated episodes can be stored in the memory, for a maximum duration of 27 and 22.5 min, respectively. When the memory is full, the new episode is stored over the record of the oldest electrocardiogram, always keeping stored at least 3 episodes of the same type.
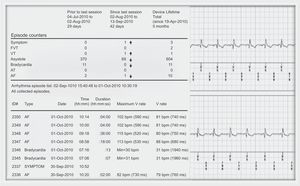
At implantation, the patient was given a CareLink® remote monitor and an “activator” to record and store symptomatic episodes. The remote monitor communicates with the ILR via an antenna that the patient must place on the skin above the ILR; when a button is pressed, the system interrogates the device and transmits the information via a telephone line to a secure web server that can be accessed by the arrhythmia unit staff for assessment. The data received through the website are identical to those obtained in an on-site visit, and are downloaded in PDF format for storage, analysis, and review (Fig. 1). The patient is instructed to call the arrhythmia unit whenever he or she notices the clinical symptoms under investigation. A technician performs an initial analysis of transmitted data, either monthly or after a symptomatic episode, and contacts the medical practitioner in the case of relevant events and the patient to correlate the electrocardiographic recordings with clinical symptoms.
Statistical AnalysisAll variables were analyzed using the SPSS 14.0 statistical package. Age and times are expressed as the median and range. Variables with a nonnormal distribution were analyzed using the Mann-Whitney U test, whereas qualitative variables with a normal distribution were analyzed by the Pearson chi-square test.
RESULTSA total of 109 patients with an ILR were followed for a mean period of 64 weeks (0.57-164.57 weeks), 41 in CF and 68 in RM. The baseline characteristics of each population group are listed in Table 2.
Characteristics of the Study Population Groups
| CF | RM | Total | |
| Patients | 41 (37.6) | 68 (62.4) | 109 (100) |
| Men | 25 (61) | 42 (61.8) | 67 (61.5) |
| Women | 16 (39) | 26 (38.2) | 42 (38.5) |
| Mean age, years | 60 (15-93) | 65.5 (17-90) | 67 (15-93) |
| Structural heart disease* | 19 (46.3) | 26 (38.2) | 45 (41.2) |
| History of syncope | |||
| <3 | 12 (29.2) | 26 (38.2) | 38 (34.8) |
| =3 | 29 (70.73) | 42 (61.7) | 71 (65.1) |
| ILR indication | |||
| Syncope | 38 (92.7) | 62 (91.2) | 100 (91.7) |
| Palpitations | 0 | 2 (2.9) | 2 (1.8) |
| Brugada syndrome | 3 (7.3) | 4 (5.9) | 7 (6.4) |
CF, conventional follow-up; ILR, implantable loop recorder; RM, remote monitoring.
For all variables compared, P>.05.
Unless otherwise indicated, data are expressed as no. (%).
The frequency of attendance at scheduled visits was analyzed according to each type of follow-up: 89.7% of patients in RM attended 0 or 1 scheduled visit; conversely, 75.6% of patients with CF attended >2 visits. Table 3 lists the types of visits and the frequency of care according to the type of follow-up. Both unexpected care (13.2% in RM vs 31.7% in CF; P=.02) and emergency room care (14.7% in RM vs 31.7% in CF; P=.03) were significantly less common in the RM group.
Significant Events Diagnosed With the Implantable Loop Recorder According to the International Study on Syncope of Unknown Etiology classification. Type of Visit During Implantable Loop Recorder Follow-up
| CF | RM | Total | P | |
| Type of ISSUE event | .97 | |||
| 1 | 11 (28.2) | 13 (25.5) | 24 (26.7) | |
| 2 | 1 (2.6) | 2 (3.9) | 3 (3.3) | |
| 3 | 21 (53.8) | 28 (54.9) | 49 (54.4) | |
| 4 | 6 (15.4) | 8 (15.7) | 14 (15.6) | |
| Type of visit | ||||
| Scheduled | <.01 | |||
| 0-1 | 10 (24.4) | 61 (89.7) | 71 (65.1) | |
| >2 | 31 (75.6) | 7 (10.3) | 38 (34.9) | |
| Emergency room | 13 (31.7) | 10 (14.7) | 23 (21.1) | .03 |
| Unscheduled | 13 (31.7) | 9 (13.2) | 22 (20.2) | .02 |
CF, conventional follow-up; ISSUE, International Study on Syncope of Unknown Etiology; RM, remote monitoring.
Data are expressed as no. (%).
In the RM group, the number of transmissions sent because of the presence of symptoms was 2.01 (2.5) and the total number of transmissions was 7.72 (5).
There were no events that had no electrocardiographic record due to ILR memory overflow in the RM group. In comparison, the ISSUE 2 study estimated that 26% of episodes reported by patients had no electrocardiographic documentation in the CF group (memory overflow and overwriting of old episodes).9
A significant event was diagnosed in 82.6% of patients with an ILR. The most common diagnostic event in both population groups (54.4% of the total) was the presence of a normal electrocardiographic record with related symptoms (ISSUE type 3) (Table 3) that led to treatment with hygiene-dietary measures in 50.4% of the entire sample. This group included all asymptomatic patients who had undergone explantation and had a normal electrocardiographic record throughout the entire follow-up. In 26.7% of the patients, the significant diagnostic event was asystole over 3 s (ISSUE type 1), leading to implantation of a PM in all such patients (24 PMs) or an ICD if there was documented ventricular tachycardia or associated ventricular dysfunction (2 ICDs), which accounted for 23.9% of all patients. All other events were treated by ablation (0.9%) or drug therapy (7.3%).
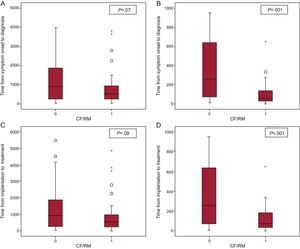
The mean time from symptom onset to ILR implantation was similar in both population groups: 366 days (2-13 900 days) for patients with CF and 369.5 days (1-23 587 days) for patients with RM. RM allowed considerably shorter mean times from symptom onset to diagnosis of a significant event (512 vs 914 days), but this difference was not statistically significant (P=.07). The mean time from ILR implantation to diagnosis of a significant event was considerable and was significantly lower in patients under RM: 56 days (0-650 days) compared with 260 days (range, 5-947 days) (P<.001) in patients under CF. As a result, RM shortened the time from device implantation to diagnosis by 204 days on average. The mean time from symptom onset to the start of treatment was shorter in the RM group—529.5 days (9-11 132 days) vs 914 days (32-14 163 days) in CF—, but not statistically significant (P=.09). The mean time from device implantation to the start of a specific treatment was significantly shorter in patients with RM: 73 days (0-650 days) vs 260 days (5-947 days) in CF (P<.001) (Fig. 2).
A: time from symptom onset to conventional follow-up/remote monitoring diagnosis. B: time from implantation to conventional follow-up/remote monitoring diagnosis. C: time from symptom onset to conventional follow-up/remote monitoring treatment. D: time from implantation to conventional follow-up/remote monitoring treatment. CF/RM, conventional follow-up/remote monitoring. Circles and asterisks refer to outliers.
The main aim of this study was to demonstrate the effectiveness of RM over time in diagnosis and specific treatment, as well as in reducing the number of scheduled and unscheduled visits, compared with CF in patients with an ILR for syncope or palpitations, without reducing health care safety and follow-up. Because the aim of this study was not to analyze the diagnostic performance of the ILR in different substrates or indications but to compare 2 follow-up strategies (on-site and remote) regardless of implant indication, a pooled analysis of the entire population was performed based on the type of follow-up, without comparative studies according to ILR indication.
In this study, in patients who performed telephone transmissions monthly or immediately in the case of manual activation, a significant event was diagnosed within a mean time of 56 days postimplantation, an average gain of 204 days compared with CF patients. Consequently, specific treatment was started an average of 187 days earlier in RM patients (mean time from ILR implantation, 73 days) than in CF patients. Because the final treatment was PM or ICD implantation in 26 of 109 patients analyzed, we consider early diagnosis and treatment to be of the utmost importance to prevent potential complications associated with a delay in establishing targeted treatment, a delay that may be due, at least in part, to episodes erased due to device memory overflow in the CF group. Moreover, in RM patients, compliance with follow-up by was probably significantly enhanced by the greater convenience associated with monthly uploads from home–which avoids 3-monthly visits–, the ease of 2-way telephone communication, and the option to analyze the data stored without requiring a clinic visit. Lastly, although the CF protocol established 3-monthly visits, the mean time between visits was 4.9 months due to the heavily burdened and overcrowded health care system. A significant diagnostic event was detected in 82.6% of all patients with an ILR, at similar percentages in both population groups, showing that RM does not increase the number of diagnoses but does shorten the time to diagnosis and allows the necessary treatment to be established as soon as possible.
The diagnostic events most commonly found in both population groups were asystole over 3 s with or without symptoms (ISSUE type 1) in 26.7% of the patients and no or slight variation in heart rate associated with symptoms (ISSUE type 3, 41.1%) or asymptomatic (13.3%) in 54.4% of the patients. We believe that a long continuous electrocardiogram monitoring period (25.4 months) showing no electrocardiogram abnormalities in patients with repeated syncope before ILR implantation allows the clinician to exclude a cardiac cause with a high negative predictive value, and, therefore, can be considered a diagnosis of a “significant event.”
Numerous studies have demonstrated the efficacy of ILR in the diagnosis of syncope; however, only the studies by Arrocha et al.17 and Furukawa et al.18 refer to remote follow-up. Additionally, telephone transmission has been thoroughly studied in other implantable electronic devices, such as PMs and ICDs.10–16 A study by Lazarus10 analyzed patients with PM and ICD who performed daily transmissions by a remote system; 86% of events were disease-related, and a decrease of 26 days in mean time to diagnosis was observed in patients under RM, compared with 64 and 154 days in patients with 3-monthly and twice-yearly visits, respectively. When the results of this study were compared with the majority of studies on ILR without RM, the mean time to diagnosis was longer in previous studies and many events were not recorded due to device memory overflow. In a pioneer study by Krahn et al.,7 the mean time to diagnosis was 5.1 (4.8) months, and in another 3 studies, the mean time was 109 (120) days,20 71 (79) days,21 and 5.4 (4.6) months,22 compared with the mean time to diagnosis (56 days) of this study. Furukawa et al.18 conducted a similar study to ours, but with no control group, that followed 47 patients for a mean of 20 (13) weeks. The patients performed weekly transmissions, achieving an estimated decrease in the mean time to diagnosis of 71 (17) days, compared with a historical control. The advantages of our study are the use of a CF control group, a larger sample size (109 patients), and a longer mean follow-up (64 weeks). The time to diagnosis with RM was decreased by 204 days with respect to CF, compared with 71 days observed by Furukawa et al.18
Although patients with RM had considerably fewer scheduled visits than patients with CF (1 to 4 visits per year), this did not lead to a higher number of unscheduled visits nor in an increase in the number of visits to the emergency room; in fact, these visits were reduced, confirming the confidence that patients showed in RM and the importance of 2-way telephone communication.
Another advantage of RM over CF is early detection of asymptomatic arrhythmic episodes, occasionally with significant clinical repercussions, with the respective specific treatment. In the case of CF, these episodes are evident at the time of the clinic visit, occasionally months after the event, and with the associated risk of loss of information due to memory overflow and episode overwriting. Automatic activation of the ILR without periodic transmission of recorded events leads to device memory overflow and detection of a higher number of false events. This had already been observed in the pilot study by Arrocha et al.,17 which followed 40 patients for 8.5 months; 223 226 electrocardiographic transmissions were analyzed at the monitoring site, of which only 117 (0.005%) were selected for further assessment. The present study achieved a large reduction in the number of transmissions by instructing patients of the need to save only symptoms analogous to those that led to implantation. The number of false arrhythmic episodes is much higher in ILR than in other devices, such as PMs or ICDs, because the ILR detects the surface electrocardiogram rather than an intracardiac signal, as in the case of other devices. A study by Heidbüchel et al.11 with ICDs showed a sensitivity of 99.5% in the detection of significant events. Another potential advantage of RM is that this load of false detections is observed earlier than with CF, with the consequential possibility of reprogramming the device to attempt to avoid false records or to increase transmission frequency and ensure that the time between them is smaller than that needed to overflow the device memory.
Finally, the lack of any increase in the number of unscheduled office or emergency room visits or any differences in the number of final significant events between the RM and CF groups confirms the safety of remote follow-up, in terms of both objective data and the patient's subjective impression.
LimitationsThis was a nonrandomized, nonprospective study, with the limitations inherent to this design, in particular a possible time and selection bias. However, because the baseline characteristics are very similar and because CF and RM were sequentially implemented at our hospital without selecting patients for either group, we believe that the validity of the data is very high.
Because of the overcrowded health care system, the actual time between visits was 4.9 months even though the CF protocol stipulated a 3-month frequency.
The significantly larger difference in the time from symptom onset to treatment in the CF group can be explained by the cumulative delay from ILR implantation to diagnosis, which is associated with a longer delay until treatment, based on the greater therapeutic capacity of the arrhythmias unit in recent years, an inherent limitation of a nonrandomized study with a historical cohort. This does not, however, explain the entire difference, which is also related to the greater proactivity and prompt reaction associated with the ILR remote follow-up protocol.
Although the direct and indirect costs of RM vs CF were not evaluated, there was a notable reduction in the number of scheduled and unscheduled cardiology outpatient visits and emergency room visits, which directly leads to lower financial costs. RM studies in patients with ICDs have shown that the total per-patient cost of follow-up is reduced by 41% (524 euros per patient).12 Cost-efficacy studies similar to these but applied to the follow-up of patients with ILR would probably yield similar results.
Although patient satisfaction with the RM system was not objectively evaluated by quality-of-life questionnaires, the decrease in the number of unscheduled and emergency room visits and patient satisfaction with telephone contact and visits suggest that patient satisfaction is favorable. Furukawa et al.18 analyzed the impact on quality of life among patients with an ILR by a questionnaire, finding that 94% of patients had no concerns about the device, 70% felt safer with continuous monitoring, and one-fifth reported that they felt sicker and considered their privacy to be invaded. All patients felt the CareLink® monitor was easy to use, capable of transmitting the data in less than 10 min, and would recommend use to other patients.
CONCLUSIONSRM enhances the diagnostic effectiveness of ILR by providing more relevant electrocardiographic recordings in shorter periods, which facilitates earlier diagnosis, leads to specific treatment, and reduces the loss of information caused by device memory overflow. This greater diagnostic effectiveness is achieved with an associated decrease in the number of outpatient, unexpected, and emergency room visits and a high degree of patient satisfaction.
CONFLICTS OF INTERESTNone declared.
.