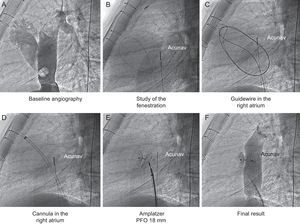
The Fontan procedure is the final step in surgery for patients with a single ventricle. The hemodynamic changes that occur after the procedure can have a negative impact on the immediate outcome due to a sudden increase in pulmonary artery pressure. Fenestration of the Fontan circuit during surgery is therefore a common procedure in high-risk patients,1 although systemic saturation may decrease as a result. The development of percutaneous implantation devices has enabled fenestration closure without the need for further surgery when hemodynamic conditions allow.2,3
Here, we analyze our experience of percutaneous closure of fenestrations in the extracardiac circuit after the Fontan procedure, taking into account the properties of the new occlusion devices available. In addition, we study the changes in pulmonary artery pressure, as well as oxygen saturation. Fourteen patients were included. Fenestration was indicated during the procedure because high-risk criteria were identified during catheterization performed prior to the Fontan procedure.4,5 One patient was excluded from the analysis because no contrast flow was observed in the baseline angiography during the procedure.
Procedures were performed under general anesthetic. An artery was cannulated (4 Fr) for monitoring. Venous cannulation was performed (6, 7, or 8 Fr) for the therapeutic intervention, which was performed with monitoring by transesophageal echocardiography in 9 cases and intracardiac echocardiography in 5. After the procedure, the therapeutic regimen comprised low-molecular-weight heparin, acetylsalicylic acid for at least 6 months, and endocarditis prophylaxis with cefuroxime axetil for 1 week.
The quantitative data were expressed as means (standard deviation). We performed a Student t test for paired data to compare the mean pulmonary pressures and systemic saturation in the same patient.
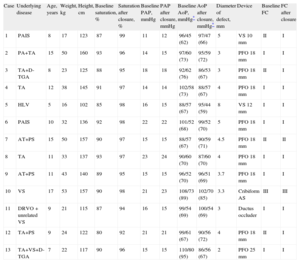
The mean (standard deviation) duration of follow-up was 45 (41) months. No major complications were recorded during the procedures. Prior therapeutic catheterization had been performed in 8 of the 13 patients to close systemic-pulmonary collaterals. Of the 13 patients analyzed, 11 had systemic left ventricle and 2 systemic right ventricle (Table). None of the patients died during follow-up or were readmitted to hospital for cardiac causes.
Characteristics of the 13 Patients
| Case | Underlying disease | Age, years | Weight, kg | Height, cm | Baseline saturation, % | Saturation after closure, % | Baseline PAP, mmHg | PAP after closure, mmHg | Baseline AoP, mmHg* | AoP after closure, mmHg* | Diameter of defect, mm | Device | Baseline FC | FC after closure |
| 1 | PAIS | 8 | 17 | 123 | 87 | 99 | 11 | 12 | 96/45 (62) | 97/47 (66) | 5 | VS 10 mm | II | I |
| 2 | PA+TA | 15 | 50 | 160 | 93 | 96 | 14 | 15 | 97/60 (73) | 95/59 (72) | 3 | PFO 18 mm | I | I |
| 3 | TA+D-TGA | 8 | 23 | 125 | 88 | 95 | 18 | 18 | 92/62 (76) | 86/53 (67) | 3 | PFO 18 mm | II | I |
| 4 | TA | 12 | 38 | 145 | 91 | 97 | 14 | 14 | 102/58 (73) | 88/57 (67) | 4 | PFO 18 mm | I | I |
| 5 | HLV | 5 | 16 | 102 | 85 | 98 | 16 | 15 | 88/57 (67) | 95/44 (59) | 8 | VS 12 mm | I | I |
| 6 | PAIS | 10 | 32 | 136 | 92 | 98 | 22 | 22 | 101/52 (68) | 99/52 (70) | 5 | PFO 18 mm | I | I |
| 7 | AT+PS | 15 | 50 | 157 | 90 | 97 | 15 | 15 | 88/57 (67) | 90/59 (71) | 4.5 | PFO 18 mm | II | II |
| 8 | TA | 11 | 33 | 137 | 93 | 97 | 23 | 24 | 90/60 (70) | 87/60 (70) | 4 | PFO 18 mm | I | I |
| 9 | AT+PS | 11 | 43 | 140 | 89 | 95 | 15 | 15 | 96/52 (70) | 96/51 (69) | 3.7 | PFO 18 mm | I | I |
| 10 | VS | 17 | 53 | 157 | 90 | 98 | 21 | 23 | 108/73 (89) | 102/70 (85) | 3.3 | Cribiform AS | III | III |
| 11 | DRVO+unrelated VS | 9 | 21 | 115 | 87 | 94 | 16 | 15 | 99/54 (69) | 100/54 (69) | 3 | Ductus occluder | I | I |
| 12 | TA+PS | 9 | 24 | 122 | 80 | 92 | 21 | 21 | 99/61 (67) | 90/56 (72) | 4 | PFO 18 mm | II | I |
| 13 | TA+VS+D-TGA | 7 | 22 | 117 | 90 | 96 | 15 | 15 | 110/80 (95) | 86/56 (67) | 2 | PFO 25 mm | I | I |
AoP, aortic pressure; AS, atrial shunt; D-TGA, D-transposition of the great arteries; DRVO, double right ventricular outflow; FC, functional class; HLV, hypoplastic left ventricle; PA, pulmonary atresia; PAIS, pulmonary atresia with intact septum; PAP, mean pulmonary artery pressure; PFO, patent foramen ovale; PS, pulmonary stenosis; SV, single ventricle; TA, tricuspid atresia; VS, ventricular shunt.
In all cases, Amplatzer occluders were used. The device type was chosen in accordance with the postsurgical anatomy, which was assessed by computed tomography prior to the procedure. Multislice computed tomography was used to locate the site of fenestration and select the angiographic view that would best visualize the defect, sliced perpendicular to the largest diameter of the defect. Once catheterization had been performed, contrast was injected and the maximum diameter of fenestration was estimated, in accordance with the calibration marked with the catheter (Figure). Computed tomography and transesophageal or intracardiac echocardiography complemented these measures and contributed to better selection of the device size. In addition to the maximum diameter of the defect, the distance between the atrial chamber and the internal edge of the conduit was taken into account. The type of Amplatzer occluder was selected in accordance with the anatomical configuration. Devices for patent foramen ovale were used for narrower fenestration while those for ventricular shunts and patent ductus arteriosus were chosen for the wider ones (Table).
In 10 cases, the device for patent foramen ovale was used (18mm in 9 patients and 25mm in 1 patient). In 2 patients, a muscular ventricular shunt device was used, while in the remaining patient, a ductus closure device was chosen. Oxygen saturation increased significantly after closure of the fenestration (89% [3.6] vs 96% [2.0]; P<.01) without any evidence of a significant increase in pulmonary artery pressure (17 [3.6] mmHg vs 17.2 [3.9] mmHg).
Patients with congenital heart disease in the form of single ventricle circulation will need to undergo surgery several times during their lives. The development of devices placed by percutaneous procedures can help avoid surgical procedures in some of these patients. Closure of the fenestration is necessary due to the long-term harmful effects of chronic hypoxemia.
The experience in our hospital suggests that a multidisciplinary approach in these patients is essential when designing therapeutic strategies and establishing the timing of the interventions. The development of percutaneous implantation devices has allowed greater flexibility in surgery, which can be adapted to the hemodynamic conditions of each patient. Closure of the Fontan fenestration by catheterization is a safe and effective technique in these patients.
We wish to acknowledge Dr. Ignacio Tejero, who passed away during the preparation of this letter. He had been involved in the treatment of these patients for past 20 years.