Hyponatremia is the most common electrolyte disturbance in patients with heart failure (HF) and, at the present time, there is no appropriate treatment strategy for its correction when associated with HF.1
The standard treatment for hypervolemia and fluid retention involves loop diuretics, drugs that produce marked natriuresis, which favors the development of hyponatremia in HF.
Arginine vasopressin concentration is known to be high in patients with decompensated HF with anasarca, which contributes to disease progression and is associated with a poorer prognosis.2 Even a slight increase in arginine vasopressin can cause considerable fluid retention, limiting urinary excretion, a circumstance that produces a state of dilution, with the resulting hyponatremia. The physiological approach to the prevention of this electrolyte disturbance consists of reducing arginine vasopressin secretion or blocking its action in the target organ. At the present time, we know of no drug capable of reducing arginine vasopressin secretion.
Tolvaptan is a new orally administered molecule that acts as a selective V2 receptor antagonist of antidiuretic hormone in the distal renal tubule3 and is designed to promote net excretion of water without electrolyte loss,4 a phenomenon known as aquaresis.
The purpose of the present report was to describe our experience in the use of tolvaptan in 10 patients to treat hyponatremia in decompensated HF. All the patients signed an informed consent form for compassionate use of the medication, as it has not been approved for use in patients with HF. In addition, all the treatments were supervised by the pharmacy service of our hospital.
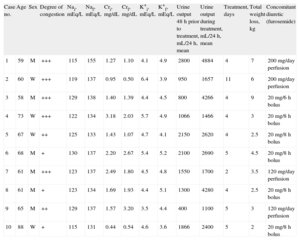
These 10 patients had hyponatremia (Na ≤ 130 mEq/L), with extracellular volume expansion or euvolemia. Tolvaptan was prescribed for its correction. In all patients, tolvaptan therapy was initiated after a period of fluid restriction of at least 48h that did not result in the correction of the hyponatremia. An analysis including the electrolyte profile and renal function parameters was performed prior to the initiation of tolvaptan therapy and daily thereafter until treatment was stopped of the aim of a sodium concentration between 135 mEq/L and 140 mEq/L was achieved. Urine output was measured over the 48h prior to initiation of tolvaptan therapy and during the treatment period. The characteristics of the patients before and after treatment are shown in the Table.
Summary of the Data Obtained in Patients Under Treatment With Tolvaptan.
| Case no. | Age | Sex | Degree of congestion | Nai, mEq/L | Naf, mEq/L | Cri, mg/dL | Crf, mg/dL | K+i, mEq/L | K+f, mEq/L | Urine output 48 h prior to treatment, mL/24 h, mean | Urine output during treatment, mL/24 h, mean | Treatment, days | Total weight loss, kg | Concomitant diuretic (furosemide) |
| 1 | 59 | M | +++ | 115 | 155 | 1.27 | 1.10 | 4.1 | 4.9 | 2800 | 4884 | 4 | 7 | 200 mg/day perfusion |
| 2 | 60 | W | +++ | 119 | 137 | 0.95 | 0.50 | 6.4 | 3.9 | 950 | 1657 | 11 | 6 | 200 mg/day perfusion |
| 3 | 58 | M | +++ | 129 | 138 | 1.40 | 1.39 | 4.4 | 4.5 | 800 | 4266 | 4 | 9 | 20 mg/6 h bolus |
| 4 | 73 | W | +++ | 122 | 134 | 3.18 | 2.03 | 5.7 | 4.9 | 1066 | 1466 | 4 | 3 | 20 mg/8 h bolus |
| 5 | 67 | W | ++ | 125 | 133 | 1.43 | 1.07 | 4.7 | 4.1 | 2150 | 2620 | 4 | 2.5 | 20 mg/8 h bolus |
| 6 | 68 | M | + | 130 | 137 | 2.20 | 2.67 | 5.4 | 5.2 | 2100 | 2690 | 5 | 4.5 | 20 mg/8 h bolus |
| 7 | 61 | M | +++ | 123 | 137 | 2.49 | 1.80 | 4.5 | 4.8 | 1550 | 1700 | 2 | 3.5 | 120 mg/day perfusion |
| 8 | 61 | M | + | 123 | 134 | 1.69 | 1.93 | 4.4 | 5.1 | 1300 | 4280 | 4 | 2.5 | 20 mg/8 h bolus |
| 9 | 65 | M | ++ | 129 | 137 | 1.57 | 3.20 | 3.5 | 4.4 | 400 | 1100 | 5 | 3 | 120 mg/day perfusion |
| 10 | 88 | W | + | 115 | 131 | 0.44 | 0.54 | 4.6 | 3.6 | 1866 | 2400 | 5 | 2 | 20 mg/8 h bolus |
+, no ankle edema or very mild edema; ++, lower limb edema; +++, anasarca; Crf, final creatinine; Cri, initial creatinine; K+f, final potassium; K+i, initial potassium; M, man; Naf, final sodium; Nai, initial sodium; W, woman.
All the patients were under treatment with intravenous furosemide prior to the initiation of tolvaptan.
Tolvaptan was administered at doses of 15mg to 30mg every 24h for a mean duration of 4.8 days (2-11 days).
After tolvaptan administration, sodium levels improved, and nearly all the patients reached the target concentration of 135 mEq/L.
We observed no complications related to tolvaptan administration, which was well tolerated in all patients. This finding has also been demonstrated in other studies.5 In 1 patient, the medication was discontinued before the treatment had been completed due to a deterioration of renal function; in another patient, the dose was reduced because of hypernatremia. Normal sodium levels were restored in this patient after reduction of the dose, and there were no clinical consequences.
Hyponatremia is associated with a poorer prognosis in HF and is an electrolyte disturbance that is difficult to treat, given that, aside from fluid restriction, there are few effective therapeutic options. Although hyponatremia is most commonly associated with congestive symptoms, it can also develop in euvolemic patients. The safety of the use of tolvaptan in these patients was demonstrated in the SALT 1 and SALT 2, which recruited both hypervolemic and euvolemic patients.
In our experience, tolvaptan is effective for the treatment of dilutional hyponatremia associated with HF and, moreover, is well-tolerated. The serum sodium level improved in all the patients studied.
In most of the patients, there was evidence of improved renal function, with a decrease in the serum creatinine and urea concentrations. In contrast, we observed no significant changes in potassium levels.
Urine output increased considerably in most patients, taking into account the fact that they were also receiving high doses of an intravenous loop diuretic. The urine output increased in variable amounts, ranging from 150mL/24h to 2900mL/24h (Table). Weight loss was more marked, in general, in the patients with the most severe congestive symptoms.
In conclusion, tolvaptan is well tolerated and is effective in correcting hyponatremia in patients with decompensated HF, without producing significant changes in other electrolytes or affecting renal function. Thus, we consider that this drug could come to be used as a first-line treatment in patients with this profile. To date, no study has demonstrated a reduction in the mortality rate or in readmissions in patients with sodium levels under 135 mEq/L,6 although a substudy of the EVEREST trial showed that tolvaptan reduces mortality and readmissions in patients with sodium levels under 130 mEq/L. Further studies will be needed to determine the effects on the long-term mortality and prognosis related to the administration of this drug in patients with HF.
.