Infections in cardiac implantable electronic devices are increasing due to the expansion of the indications of these devices. The management of some aspects is controversial. Here, we report our broad experience.
MethodsBetween 1985 and 2015, 325 infections (196 local and 129 systemic) were registered; 28.5% of them were referred from other centers: 229 pacemakers, 69 implantable cardioverter-defibrillators, and 27 patients with cardiac resynchronization therapy. The follow-up was at least 1 year after hospital discharge.
ResultsPercutaneous traction (PCT) was the most frequent procedure (n=280) in local (n=166) and systemic infections (n=114), with complete extraction of the system in 82.5% of the patients, clinical success in 89%, and few complications (2 deaths attributable to the technique). Overall mortality was 1% in local infections and 8% in systemic infections. After 212 complete PCT, a new device was placed in 209: of these, a contralateral system was implanted in the same procedure in 152 (73%) and in a second procedure in 57, with no differences in relapses (2 in the 1-stage procedure, and 1 in the 2-stage procedure).
ConclusionsPercutaneous traction in experienced hands has good results with very few complications. It is possible to perform contralateral implantation of the new device on the same day without increasing the risk of relapse.
Keywords
The first permanent pacemaker implantation was performed in 1954, with the first implantable cardioverter defibrillator implantation occurring in 1980. The use of these cardiac implantable electronic devices (CIEDs) has exponentially increased in the last 10 years due to technological advances, expansion of their indications, and improved life expectancy of the recipient population.1–3 This increment has been accompanied by a largely unforeseen increase in infectious complications affecting the devices.4 The treatment of these complications is complicated and, despite the existence of specific management guidelines,5,6 certain aspects related to diagnosis and particularly treatment are still a matter of debate. The aim of this work was to describe our broad experience in the treatment of these infections, primarily by analyzing the success of the various therapeutic approaches used.
METHODSStudy Population and PeriodThe present study analyzed consecutive patients with CIED infections treated between 1985 and 2015 in our center. From 1985 to 1998, the patients were referred from the clinic to the infectious disease service. In 1999, a specific intervention program developed by a multidisciplinary team was implemented for these types of infections. When a CIED infection was suspected, the patients (from the hospital itself or transferred from other centers) were evaluated by a team member (A. de Alarcón González) to determine the type of infection and the priority for action. Data were prospectively and systematically collected according to a preestablished protocol.
Microbiological Sample Collection and Echocardiography ResultsTwo batches of serial blood cultures (baseline and at 30 and 60minutes) were obtained from all patients at a 12-hour interval. If patients showed local signs of infection, the exudate was sampled for Gram staining and aerobic and anaerobic cultures; if there was no external discharge, sampling was performed using needle aspiration of the generator pocket. During the surgery, samples were obtained from the pocket and the region 4cm distal to the leads; some of the samples were cultured while the remainder was stored at –20°C. From 2005, negative cultures were additionally analyzed with genomic sequencing techniques, specifically, 16S rRNA polymerase chain reaction (PCR). If systemic infection was suspected and/or the blood cultures were positive, cardiac ultrasound was performed (in transesophageal mode from 1999 onward).
DefinitionsInfections were classified according to location as:
- •
Local infection: infections were considered local when patients showed no systemic symptoms (fever, shock, embolisms, or remote infectious complications), blood cultures were negative, and there were signs of infection in the region of the generator pocket, such as pain, erythema, and purulent material.
- •
Systemic infection: infections were considered systemic when patients showed systemic symptoms and blood cultures were repeatedly positive. Negative blood cultures required the presence of vegetation in the lead or right-sided cardiac structures.
Depending on the time of onset, infections were classified as: a) acute: those appearing before 1 month after device implantation or manipulation; b) delayed: those appearing between 2 and 12 months after implantation or manipulation; and c) late: those appearing after 12 months.
Antibiotic TherapyAntibiotic therapy was always performed in accordance with the antibiogram of the causative agent isolated in culture. After device removal, oral antibiotic therapy continued for 1 or 2 weeks for local infections. If the device was not removed, the duration varied from patient to patient.
Initial treatment of systemic infections always involved intravenous antibiotics before device removal; except in patients with severe sepsis, the device was not removed until the blood cultures were negative (typically at 1 week). If the removal was complete, the therapy was maintained for 2 further weeks and, after the first week, could be switched to oral therapy. If valves were affected (eg, the tricuspid), the antibiotic therapy was maintained for at least 4 weeks.
Surgical ProcedureThe surgical procedures are detailed in the supplementary material.
Replacement of the Implantable Electronic DeviceDevice replacement was performed in 1 or 2 stages, depending on the type of device, as described in the supplementary material.
Follow-upPatients of our hospital underwent a specific follow-up for 1 year in a clinic specializing in infectious diseases and then for life in a pacemaker assessment clinic. Patients referred from other centers underwent a minimum follow-up for 3 months in our hospital; all such patients also underwent follow-up blood cultures for systemic infections and stayed in contact with the referral hospital for 1 year.
Infection relapse was considered to have occurred if, during the 1-year follow-up, the newly implanted device was infected by the same microorganism isolated from the originally extracted system. In contrast, the infection was considered to be a reinfection if a different microorganism was isolated; it was then recorded as a new episode. Persistence of the original signs and symptoms or continued isolation of the same microorganism after a specific therapeutic intervention was defined as treatment failure.
Statistical AnalysisA descriptive analysis with measures of central tendency and dispersion was performed for continuous variables, as well as a frequency distribution for qualitative variables. For the comparison of continuous variables, the Student t test was used (after analysis of normality with the Kolmogorov-Smirnov test); the Mann-Whitney U test was used if the distribution was not normal. For categorical variables, the chi-square test or Fisher exact test was used as appropriate. P < .05 was considered statistically significant.
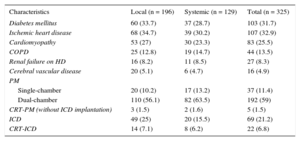
RESULTSEpidemiological CharacteristicsDuring the study period, 325 infections were recorded in 314 patients, 226 (72%) men and 88 women; 28.5% of the patients had been referred from other centers. The median patient age was 69 [60-76] (range, 14-90) years and 32% had 2 or more concomitant chronic diseases.
The infection was related to a pacemaker in 229 patients (71%), to an implantable cardioverter defibrillator in 91, and to cardiac resynchronization therapy in 27 (22 had received an implantable cardioverter defibrillator). The main characteristics of the patients are summarized in Table 1.
Epidemiological Characteristics of Patients by Type of Infection
| Characteristics | Local (n = 196) | Systemic (n = 129) | Total (n = 325) |
|---|---|---|---|
| Diabetes mellitus | 60 (33.7) | 37 (28.7) | 103 (31.7) |
| Ischemic heart disease | 68 (34.7) | 39 (30.2) | 107 (32.9) |
| Cardiomyopathy | 53 (27) | 30 (23.3) | 83 (25.5) |
| COPD | 25 (12.8) | 19 (14.7) | 44 (13.5) |
| Renal failure on HD | 16 (8.2) | 11 (8.5) | 27 (8.3) |
| Cerebral vascular disease | 20 (5.1) | 6 (4.7) | 16 (4.9) |
| PM | |||
| Single-chamber | 20 (10.2) | 17 (13.2) | 37 (11.4) |
| Dual-chamber | 110 (56.1) | 82 (63.5) | 192 (59) |
| CRT-PM (without ICD implantation) | 3 (1.5) | 2 (1.6) | 5 (1.5) |
| ICD | 49 (25) | 20 (15.5) | 69 (21.2) |
| CRT-ICD | 14 (7.1) | 8 (6.2) | 22 (6.8) |
COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HD, hemodialysis; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
Data are expressed as n (%).
The infections were categorized as local in 196 episodes (60.3%) and systemic in 129 (39.7%). In 160 patients (49%), the infection developed after further manipulation (generator replacement in 80% of these infections). The infections were classified as acute (n = 131 [40.3%]), delayed (n = 100 [30.8%]), and late (n = 94 [28.9%]).
Etiology and Yield of the Diagnostic TestsThe most frequently isolated microorganisms were coagulase-negative staphylococci (42.8%) and Staphylococcus aureus (16.9%). A more detailed description of the microbial etiology can be found in Table 2A and Table 2B.
Etiology of the Infections
| A. According to time of onset | ||||
|---|---|---|---|---|
| Microorganism | Acute | Delayed | Late | Total |
| CNSa | 48 (36.6) | 46 (46) | 45 (47.9) | 139 (42.8) |
| Staphylococcus aureusb | 30 (22.9) | 13 (13) | 12 (12.8) | 55 (16.9) |
| GNB | 16 (12.2) | 4 (4) | 4 (4.3) | 24 (7.3) |
| Streptococcus | 1 (0.8) | 1 (1) | 4 (4.3) | 6 (1.8) |
| Anaerobic | 6 (6.6) | 5 (5) | 2 (2.1) | 13 (4) |
| Other | 2 (1.5) | 4 (4) | 5 (5.3) | 12 (3.6) |
| Polymicrobial | 16 (12.2) | 9 (9) | 9 (8.5) | 33 (10.2) |
| Unknown | 12 (9.2) | 18 (18) | 13 (13.8) | 43 (13.2) |
| Total | 131 | 100 | 94 | 325 |
| B. According to location | |||
|---|---|---|---|
| Microorganism | Local | Systemic | Total |
| CNSa | 90 (45.9) | 49 (38) | 139 (42.8) |
| Staphylococcus aureusb | 20 (10.2) | 35 (27.1) | 55 (16.9) |
| GNB | 13 (8.6) | 11 (8.5) | 24 (7.3) |
| Streptococcus | 1 (0.5) | 5 (3.9) | 6 (1.8) |
| Anaerobicc | 13 (6.6) | 0 | 13 (4) |
| Otherd | 6 (3.1) | 6 (4.5) | 12 (3.6) |
| Polymicrobiale | 12 (6.1) | 22 (16.3) | 33 (10.2) |
| Unknown | 41 (20.9) | 2 (1.6) | 43 (13.2) |
| Total | 196 | 129 | 325 |
CNS, coagulase-negative staphylococci; GNB, Gram-negative bacillus (including enterobacteria and non-fermenting bacilli).
In 11 CNS-related infection episodes, various species were isolated that have been omitted from the “polymicrobial” category.
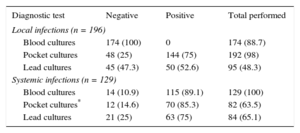
When there were local symptoms, the culture yield of the generator region was high (75.0%-85.3% of isolations); also high were the culture positivity of the leads in systemic infections (75%) and even in local infections (52.6%). A more detailed summary of these results is shown in Table 3.
Yields of the Various Cultures Performed by Type of Infection
| Diagnostic test | Negative | Positive | Total performed |
|---|---|---|---|
| Local infections (n = 196) | |||
| Blood cultures | 174 (100) | 0 | 174 (88.7) |
| Pocket cultures | 48 (25) | 144 (75) | 192 (98) |
| Lead cultures | 45 (47.3) | 50 (52.6) | 95 (48.3) |
| Systemic infections (n = 129) | |||
| Blood cultures | 14 (10.9) | 115 (89.1) | 129 (100) |
| Pocket cultures* | 12 (14.6) | 70 (85.3) | 82 (63.5) |
| Lead cultures | 21 (25) | 63 (75) | 84 (65.1) |
Data are expressed as n (%).
An echocardiographic study before device removal was performed in 116 patients (90%) with systemic infection: 45 patients underwent a transthoracic study, 42 underwent a transthoracic and transesophageal study, and 29 proceeded directly to a transesophageal study. Of the 87 transthoracic ultrasound studies, vegetation was visible in 24 (27.5%) vs 63.3% (n = 45) of the 61 transesophageal studies.
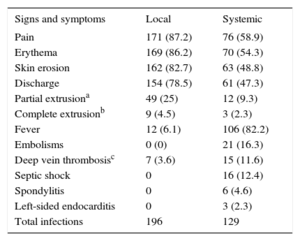
Clinical ManifestationsThe clinical signs and symptoms are shown in Table 4. Of the patients with systemic infection, 77 (57.7%) had local signs; because these signs were present a long time before the onset of the systemic infection in 32 (25%) patients, they were given standard therapy for local infection (typically with local septic surgery) before the disease progressed to a systemic infection.
Clinical Manifestations
| Signs and symptoms | Local | Systemic |
|---|---|---|
| Pain | 171 (87.2) | 76 (58.9) |
| Erythema | 169 (86.2) | 70 (54.3) |
| Skin erosion | 162 (82.7) | 63 (48.8) |
| Discharge | 154 (78.5) | 61 (47.3) |
| Partial extrusiona | 49 (25) | 12 (9.3) |
| Complete extrusionb | 9 (4.5) | 3 (2.3) |
| Fever | 12 (6.1) | 106 (82.2) |
| Embolisms | 0 (0) | 21 (16.3) |
| Deep vein thrombosisc | 7 (3.6) | 15 (11.6) |
| Septic shock | 0 | 16 (12.4) |
| Spondylitis | 0 | 6 (4.6) |
| Left-sided endocarditis | 0 | 3 (2.3) |
| Total infections | 196 | 129 |
Data are expressed as n (%).
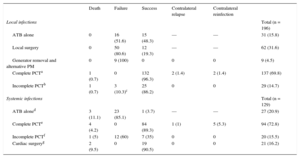
In 102 local infections (55.6%), the therapeutic approach of choice involved various types of percutaneous traction (PCT). Unsatisfactory results were obtained in 75 (73.5%). Local surgery (generator removal with the leads left in situ, pocket debridement and cleaning, and placement of a new generator) had a clinical success rate of just 20%, with a median of 2.5 [1-3] (1-12) procedures per patient. The cables were left in place on 9 occasions, even though a system was implanted contralaterally to the infected generator; in all patients, extrusion and discharge were witnessed in the same region months afterward. Isolated antimicrobial therapy was successful on 48.3% of occasions. A comparison of the patients successfully treated with this therapy (n = 15) with those who were not (n = 16) revealed the following: a) the patients had a significantly shorter median clinical course: 10 [3-15] (2-60) days vs 35.5 [16-187] (11-474) days (P < .01), and b) the patients less frequently developed skin erosion: 4 occasions (28.6%) vs 11 (64.7%; P = .07). In both situations, the prescribed antimicrobial therapy was prolonged—60 [40-90] days—and was always guided by the antibiogram.
In contrast, PCT ultimately achieved clinical success in 96.3% of complete removals (n = 94) and in 86.2% of incomplete removals (n = 20) due to retained intravascular fragments after lead rupture. In 3 cases, the patients developed, months later, a systemic infection that ultimately led to open heart surgery.
The antimicrobial therapy had poor efficacy for systemic infections (an 85.1% failure rate) and a high mortality rate (11.1%). Complete PCT achieved a clinical success rate of 89.3%, whereas incomplete PCT had a failure rate of 60% (n = 12), leading to cardiac surgery in 11 patients and subsequent femoral snare removal in the remainder. Of the 21 patients who underwent cardiac surgery in our center, 11 required it for PCT failure; the remainder had been referred from other centers that had tried simple manual traction. The results of the various approaches are detailed in Table 5.
Local and Systemic Infection Outcomes by Type of Approach
| Death | Failure | Success | Contralateral relapse | Contralateral reinfection | ||
|---|---|---|---|---|---|---|
| Local infections | Total (n = 196) | |||||
| ATB alone | 0 | 16 (51.6) | 15 (48.3) | — | — | 31 (15.8) |
| Local surgery | 0 | 50 (80.6) | 12 (19.3) | — | — | 62 (31.6) |
| Generator removal and alternative PM | 0 | 9 (100) | 0 | 0 | 0 | 9 (4.5) |
| Complete PCTa | 1 (0.7) | 0 | 132 (96.3) | 2 (1.4) | 2 (1.4) | 137 (69.8) |
| Incomplete PCTb | 1 (0.7) | 3 (10.3)c | 25 (86.2) | 0 | 0 | 29 (14.7) |
| Systemic infections | Total (n = 129) | |||||
| ATB aloned | 3 (11.1) | 23 (85.1) | 1 (3.7) | — | — | 27 (20.9) |
| Complete PCTe | 4 (4.2) | 0 | 84 (89.3) | 1 (1) | 5 (5.3) | 94 (72.8) |
| Incomplete PCTf | 1 (5) | 12 (60) | 7 (35) | 0 | 0 | 20 (15.5) |
| Cardiac surgeryg | 2 (9.5) | 0 | 19 (90.5) | 0 | 0 | 21 (16.2) |
ATB, antibiotic; PCT, percutaneous traction; PM, pacemaker.
Data are expressed as n (%).
83 PCTs as initial technique and 54 as secondary technique after failure of other approaches (antibiotic treatment alone or in combination with local surgery).
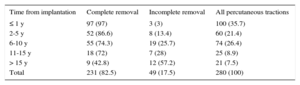
Percutaneous traction was performed for a total of 280 episodes (166 local infections and 114 systemic infections); complete removal was achieved in 231 patients (82.5%) and a cure was achieved in 246 (89%). The success rate of this technique was related to the age of the implanted CIED (Table 6), with no influence of other factors such as the number of leads, implantation side, and type of device.
Percutaneous Traction Results by Time From Implantation
| Time from implantation | Complete removal | Incomplete removal | All percutaneous tractions |
|---|---|---|---|
| ≤ 1 y | 97 (97) | 3 (3) | 100 (35.7) |
| 2-5 y | 52 (86.6) | 8 (13.4) | 60 (21.4) |
| 6-10 y | 55 (74.3) | 19 (25.7) | 74 (26.4) |
| 11-15 y | 18 (72) | 7 (28) | 25 (8.9) |
| > 15 y | 9 (42.8) | 12 (57.2) | 21 (7.5) |
| Total | 231 (82.5) | 49 (17.5) | 280 (100) |
Data are expressed as n (%).
In the entire series, there were 12 deaths: 2 patients (1%) with local infection and 10 (7.7%) with systemic infection. Only 2 of these deaths were related to the PCT technique (superior vena cava tear: 1 in a patient with local infection and 1 with systemic infection). In another 7 episodes, the cause was the sepsis itself: 3 of these patients were undergoing antimicrobial therapy, 2 underwent PCT (1 with incomplete removal and 1 with complete removal), and 2 underwent cardiac surgery. Another 2 patients died several days after PCT completion from unrelated causes (stroke, 1 of them with local infection) and, finally, another patient with dilated cardiomyopathy who underwent resynchronization therapy but did not have system replacement died months afterward due to heart failure.
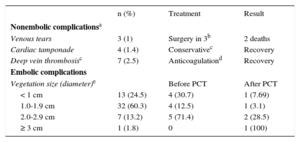
The various PCT-related complications are described in Table 7.
Percutaneous Traction Complications
| n (%) | Treatment | Result | |
|---|---|---|---|
| Nonembolic complicationsa | |||
| Venous tears | 3 (1) | Surgery in 3b | 2 deaths |
| Cardiac tamponade | 4 (1.4) | Conservativec | Recovery |
| Deep vein thrombosisc | 7 (2.5) | Anticoagulationd | Recovery |
| Embolic complications | |||
| Vegetation size (diameter)e | Before PCT | After PCT | |
| < 1 cm | 13 (24.5) | 4 (30.7) | 1 (7.69) |
| 1.0-1.9 cm | 32 (60.3) | 4 (12.5) | 1 (3.1) |
| 2.0-2.9 cm | 7 (13.2) | 5 (71.4) | 2 (28.5) |
| ≥ 3 cm | 1 (1.8) | 0 | 1 (100) |
PCT, percutaneous traction.
In the 59 episodes (45% of all systemic infections) with vegetation, the vegetation was localized to the leads (n = 45), the leads and the right valves (n = 8), and the valves alone (n = 8). The vegetation diameter was measured in 57 episodes and was < 1cm in 13 patients, 1.0-1.9cm in 34, 2.0-2.9cm in 9, and≥ 3 cm in 1.
Device replacement after its removal was not performed in 13 patients (4%) in this series.
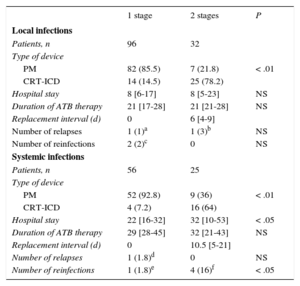
In 9 patients with local infections who underwent PCT with complete device removal, a contralateral implantation was first performed and the generator alone was removed; in our center, the remaining leads were removed because the patients continued to show signs of infection. Of the 128 remaining patients, the replacement was performed in the same surgical session in 96 (75%) and in 2 stages in 32, with no significant differences in contralateral infection frequency.
In the 4 patients with systemic infection who underwent PCT, the removal was performed after placement of a contralateral system. Of the 81 remaining replacements, the replacement was performed at the same time as the removal in 56 (69%) and at a later stage in 25. There was 1 infection by the same microorganism and another by a different bacterium (3.5%) in 1-stage procedures, whereas there were 4 infections (16%) by a different microorganism in 2-stage procedures (P = .04).
The differences between the 2 procedures are shown in Table 8.
Characteristics of the Patients Who Underwent Percutaneous Traction and Replacement in 1 or 2 Stages
| 1 stage | 2 stages | P | |
|---|---|---|---|
| Local infections | |||
| Patients, n | 96 | 32 | |
| Type of device | |||
| PM | 82 (85.5) | 7 (21.8) | < .01 |
| CRT-ICD | 14 (14.5) | 25 (78.2) | |
| Hospital stay | 8 [6-17] | 8 [5-23] | NS |
| Duration of ATB therapy | 21 [17-28] | 21 [21-28] | NS |
| Replacement interval (d) | 0 | 6 [4-9] | |
| Number of relapses | 1 (1)a | 1 (3)b | NS |
| Number of reinfections | 2 (2)c | 0 | NS |
| Systemic infections | |||
| Patients, n | 56 | 25 | |
| Type of device | |||
| PM | 52 (92.8) | 9 (36) | < .01 |
| CRT-ICD | 4 (7.2) | 16 (64) | |
| Hospital stay | 22 [16-32] | 32 [10-53] | < .05 |
| Duration of ATB therapy | 29 [28-45] | 32 [21-43] | NS |
| Replacement interval (d) | 0 | 10.5 [5-21] | |
| Number of relapses | 1 (1.8)d | 0 | NS |
| Number of reinfections | 1 (1.8)e | 4 (16)f | < .05 |
ATB, antibiotic; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; PCT, percutaneous traction; PM, pacemaker.
Data are expressed as n (%) or median [interquartile range].
Of the 304 surviving patients, 13 (4.3%) did not complete the minimum follow-up (3 months). The others were followed up for at least 1 year—median 36 [9.5-70.5] (6-309) months—, although 12 patients had not reached this time point by study closure. There were 31 deaths (10%) due to causes unrelated to device problems.
DISCUSSIONThere are few reports on CIED infections in Spain,7–9 although the general consensus is clear on the need to completely remove these devices when they are infected.5,6 However, this attitude is not shared by all members of our community, and a surprisingly high number of local surgeries are performed (even several in the same patient), even though the clinical success rate with this procedure is less than 20%. Therefore, it is not particularly surprising that up to 25% of systemic infections recorded in this series were due to a local infection that was treated with an inadequate approach. The reasons for this poor management could be a lack of recognition of the infection and an inadequate preparation for complete device removal. However, when correctly performed, culture sampling provides highly pertinent information; in this regard, data on the efficacy of antimicrobial therapy for local infections are particularly interesting when culture sampling is performed in favorable circumstances.
The best treatment for any local infection is complete device removal, but 50% of infections develop after a manipulation (typically, generator replacement)10,11 of a device with years-old leads. In this situation, unless a team trained in device removal is available, appropriate and early antimicrobial therapy can resolve the problem in certain selected patients. In contrast, in systemic infections, the aim should always be complete removal because, in our experience, isolated antimicrobial therapy leads to failure or death in 96% of events. In the literature, there are examples of cures with antimicrobial therapy alone but only 1 study endorses this protocol-based alternative in patients without symptoms at the generator or visible vegetation in the transesophageal ultrasound.12 In our series, only 4 patients fulfilling this condition were exclusively treated with antimicrobials but all showed infection relapse; consequently, our center abandoned this approach, which has even been linked to higher mortality.13 Thus, PCT performed by experienced operators is the technique of choice and we do not believe that its rejection is justified even in patients with vegetation > 2cm due to embolism risk, as recommended in some reports.14,15 In this study, 3 patients with vegetation > 2cm who underwent PCT had symptoms of pulmonary embolism after device removal but recovered quickly. This finding is in line with that of other authors who, without denying the higher number of embolizations with a higher vegetation diameter, failed to observe that the diameter influenced the final outcome or the duration of hospital stay of the patients.16
Cardiac surgery should be considered a secondary option due to its high mortality rate (10% in this series), which is not surprising given the age and comorbidities of most of these patients.17 Neither is PCT free from major, potentially fatal, complications (2 patients in this study). Even when performed by experienced operators and under optimal safety conditions,18 there is a need for the support of cardiac surgeons well-acquainted with the technique to immediately act if major complications develop. The success of PCT largely depends on operator experience and lead age.19 In local infections, even an incomplete removal can achieve a cure.20 However, notably, on 3 occasions in this series, an incomplete extraction for a local infection led to a systemic infection months later. In fact, the classification of local and systemic infections based on blood culture positivity and/or detection of lead vegetation seems reasonable but the lead culture was positive in 50% of the patients with infections categorized as “local” in this series. One possible explanation for this finding is that upon PCT removal of the lead, it passes through the infected area at the generator pocket.21 However, up to 72% of the intravascular portion of leads extracted via the femoral route from patients with infection categorized as strictly local have positive cultures.22 Thus, the difference between local and systemic infection is sometimes unclear and should always prompt the removal of all material, regardless of infection type.
Our study found interesting results regarding the frequent performance of implantation replacement in a single stage. This approach reduced the length of hospital stay and optimized operating room use. In a survey performed in Europe, most centers implanted devices in a second stage with an interval ranging from 48hours to 2 weeks depending on the infection type23; in this period, temporary pacemakers were inserted via jugular, femoral, or epicardial access. In fact, the clinical practice guidelines recommend device removal with a temporary pacing system and implantation of the new definitive system if the blood cultures are negative at 72hours.5,6 However, the value of this “expert recommendation” for local infections has recently been questioned, with Mountantonakis et al.24 showing that replacement in a 1-stage intervention is not associated with a higher incidence of reinfections. In systemic infections, the reluctance to perform the replacement at the same time as the removal is even greater because it is assumed that replacement in the same intervention could contaminate the new system with the old. However, antimicrobial therapy strongly affects the adhesion of microorganisms to abiotic materials, even at suboptimal concentrations, as shown in in vitro studies25 and experimental models.26 Thus, there is no convincing reason to believe that there is a higher risk of infection of the new device in a patient receiving appropriate antimicrobial therapy and with negative blood cultures who undergoes complete removal of the infected material. This has been shown in other implants27 and, in fact, in our series, there was no association between 1-stage replacement and a higher reinfection rate. In fact, when these procedures were split into 2 stages, there was a higher rate of infection of the new device by microorganisms resistant to the administered therapy. Long-term antimicrobial therapy might favor greater skin colonization by species naturally insensitive to the antibiotic. Other authors21,28 also believe that the time to replacement should be reduced when blood cultures are negative.
LimitationsOur work has some limitations: a) this study was performed in a referral center and there may have been an excessive number of local septic surgery failures because most patients referred to us had already undergone this failed approach, which means that the data fail to reflect its real efficacy; however, the analysis was repeated with patients from our center alone (data not shown) and the results were identical; b) because a marked percentage of patients were referred from other centers, the positive results of the microbiological studies shown here might be even lower than the actual figures, given that in many cases the cultures were not correctly obtained in the original hospital and the patients were already undergoing antimicrobial therapy, greatly complicating the identification of the causative agent; similarly, because a transesophageal echocardiographic study was not performed in all patients with systemic infections, the number of vegetations might actually be higher than that reported here; however, we do not believe that this would markedly affect the fundamental crux of the study, which is analysis of the various therapeutic approaches; and c) replacement in 1 or 2 stages was not random, with a greater number of defibrillator and resynchronization implants in the 2-stage replacement group; due to their greater size and the increased implantation difficulty, the surgical procedure is longer and favors a greater number of infections. However, this does not invalidate the conclusion that the 1-stage replacement is equally as safe and avoids the inconvenience of the “bridge interval” in pacemaker-dependent patients (bedridden and long-term hospitalized).
CONCLUSIONSAlthough CIED infections are an emerging problem, they are sometimes poorly recognized and treated in Spain. However, PCT in experienced hands can achieve satisfactory results and largely avoid the need for cardiac surgery. One-stage replacement does not appear to be inferior to 2-stage replacement.
CONFLICTS OF INTERESTNone declared.
- –
Although CIED infections are an emerging problem, they are not always adequately diagnosed.
- –
Despite the existence of guidelines and recommendations on their treatment, there are serious doubts about some aspects of the indicated treatment (duration, administration route, efficacy of percutaneous removal) and the time of replacement.
- –
Few studies have been published on the topic in Spain.
- –
Our work comprises an extensive series collected from a single center and with a uniform methodology.
- –
The article details the microbial etiology and the yield of the diagnostic techniques, analyzes the distinct efficacies of the diverse therapeutic procedures available, and addresses the problem of device replacement.
- –
Our results robustly support the use of PCT and 1-stage device replacement.
The authors are indebted to Dr. Enrique Oliva de Anquín, whose early guidance and enthusiastic support made this program possible.