Keywords
INTRODUCTION
Platelet aggregation and thrombosis play a major role in the development of acute coronary syndromes (ACS). Treatment with aspirin and heparin is recommended in all patients with acute myocardial infarction and unstable angina,1-4 while fibrinolysis remains the most frequently used method of reperfusion therapy in patients with acute ST-segment elevation myocardial infarction (STEMI),5,6 and glycoprotein IIb/IIIa inhibitors are frequently used in conjunction with percutaneous coronary intervention (PCI). As a result, an increasing number of patients receive several antithrombotic drugs simultaneously during the first few days after developing unstable angina or suffering a myocardial infarction. The potential increase in bleeding risk associated with combination therapy has not been fully investigated in clinical practice. The use of thienopyridines, in particular clopidogrel, in ACS has been associated with an improvement in outcome.7-10 However, little information is available about the use of thienopyridines outside the context of clinical trials. The outcome in regular patients is unknown and concern exists regarding the potential increase in bleeding risk.
The aim of this study was to analyze the rates of major bleeding and in-hospital death in patients with STEMI who were treated with thienopyridines, with or without fibrinolytic drugs, in an unselected population of patients enrolled in the Global Registry of Acute Coronary Events (GRACE).
METHODS
Study Population
The GRACE study is a large, prospective, multinational, observational study designed to reflect an unbiased population of patients with the spectrum of ACS.11,12 A total of 106 hospitals in 14 countries have contributed data to the study. Patients entered in the registry had to be at least 18 years old and alive at the time of hospital presentation, be admitted for ACS as a presumptive diagnosis (ie, to have symptoms consistent with acute ischaemia) and have at least one of the following: electrocardiographic changes consistent with ACS, serial increases in serum biochemical markers of cardiac necrosis, and/or documentation of coronary artery disease. The qualifying ACS must not have been precipitated by significant non-cardiovascular comorbidity such as trauma or surgery.
To ensure the enrolment of an unbiased population, the first 10-20 consecutive patients were recruited from each study site on an ongoing monthly basis. Data were collected by trained coordinators using a standardized case report form. Demographic characteristics, medical history, symptoms at presentation, biochemical and electrocardiographic findings, treatment practices, and a variety of hospital outcome data were collected. In-hospital management of the patients was left to the discretion of the investigating physicians. Standardized definitions for all patient-related variables and clinical diagnoses were used.11,13 STEMI was defined by the presence of new ST-segment elevation >0.1 mV in 2 contiguous leads in the index or qualifying electrocardiogram, and serum biochemical markers indicative of myocardial necrosis within each hospital laboratory's normal range. Patients originally admitted for unstable angina but in whom STEMI occurred during the hospital stay were classified as having STEMI. Patients with STEMI who were transferred from hospitals not involved in the GRACE registry were excluded.
Patients were divided into 4 groups according to the treatment they received during the first 24 h of admission or at any time during the index hospitalization: a) thienopyridines (clopidogrel or ticlopidine) without fibrinolytic drugs; b) fibrinolytic drugs without thienopyridines; c) both thienopyridines and fibrinolytic drugs; and d) neither thienopyridines nor fibrinolytic drugs.
Clinical Endpoints
The principal endpoints of this study were inhospital major bleeding and all-cause mortality. Major bleeding was defined as life-threatening bleeding with at least 1 of the following present: a bleed requiring a transfusion of 2 or more units of packed red cells, or a bleed resulting in an absolute reduction in hematocrit of ≥10%.11 Major bleeding also included intracranial bleeding or bleeding that resulted in death. Bleeding episodes that occurred secondary to coronary artery bypass surgery were excluded. Although the full spectrum of renal dysfunction is related to a higher risk for bleeding and outcome, for this study severe renal dysfunction was arbitrarily defined as a creatinine clearance of <30 ml min p
Statistical Analysis
Comparisons between groups were made using the 2-tailed Kruskal-Wallis test for continuous variables and the χ2 or Fisher's exact test for categorical variables. Multivariable logistic regression analysis was carried out to identify factors associated with the hospital use of thienopyridine, adjusting for a variety of demographic and clinical factors.
The study population was divided into 2 groups according to whether or not they received a fibrinolytic drug. The relationship between thienopyridine use and major bleeding episodes was examined in both groups after adjusting for cardiac catheterization and PCI a) at any time during hospitalization; b) during the first 24 h; and c) after the first 24 h, and for the variables included in the GRACE model for bleeding (age, sex, weight, use of fibrinolytic drugs, history of bleeding, prior anticoagulation, use of any heparin, aspirin or glycoprotein IIb/IIIa inhibitors, and renal dysfunction).14
The relationship between treatment with various medications and in-hospital mortality was also examined in both groups after adjusting for cardiac catheterization and PCI a) at any time during hospitalization; b) during the first 24 h; and c) after the first 24 h, and for the variables included in the GRACE in-hospital mortality model (age, Killip class, systolic blood pressure, ST-segment deviation, cardiac arrest during presentation, serum creatinine level, positive initial cardiac enzyme findings, and heart rate).15
All tests were 2 sided; significance was set at a≤0.05. Because of the multiple comparisons and the exploratory nature of these analyses, results of borderline statistical significance (α= .01-.05) were considered as hypothesis-generating and not definitive.
RESULTS
Thienopyridine Use in Patients With STEMI
A total of 14 259 patients with STEMI at admission were included in this analysis. These patients were admitted to GRACE hospitals between April 1999 and December 2005. Overall, 70% of the study population were men. The median age of the patients was 65 years. Some 7384 patients (52%) received thienopyridines at some time (5239 during the first 24 h) and 5051 (35%) received fibrinolytic drugs (1093 [21%] with thienopyridines within the first 24 h and 2044 [41%] with thienopyridines at some time during hospitalization). Over the study period the use of thienopyridines increased markedly, from 32% in 1999 (clopidogrel 19%; ticlopidine 15%) to 70% (clopidogrel 69%; ticlopidine 4.4%) in 2005. The most commonly used fibrinolytic drug was alteplase (57%), followed by streptokinase (33%), tenecteplase (8.5%), and other fibrinolytic drugs (1.5%).
Patient Characteristics (Table 1)
Patients who received thienopyridines alone presented with a similar clinical history and admission profile to patients receiving fibrinolytic drugs alone or in combination with thienopyridines. Treatment during hospitalization was similar in these groups, with the exception of the use of glycoprotein IIb/IIIa inhibitors, statins, and PCI, each of which was more commonly used in patients receiving thienopyridines. Patients who did not receive fibrinolytic drugs or thienopyridines had a higher risk profile both in terms of the risk of bleeding and death during hospitalization. These patients were characterized as being older and included a greater proportion of those with previous angina and myocardial infarction, diabetes, renal dysfunction, chronic heart failure and stroke, and a poorer Killip class at admission. In addition, patients who did not receive either thienopyridines or fibrinolytic drugs were less likely to receive evidence-based cardiac therapies during hospitalization including, aspirin, beta-blocker, and statin therapies.
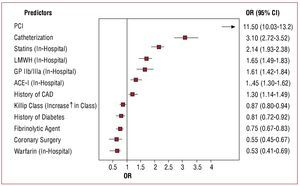
After multivariable analysis, the use of thienopyridines was associated with a medical history of coronary artery disease, cardiac catheterization, the in-hospital use of angiotensin-converting enzyme inhibitors, statins, low molecular weight heparin and glycoprotein IIb/IIIa inhibitors, and especially with PCI (Figure).
Figure 1. Multivariate analysis predicting in-hospital use of thienopyridines in patients with ST-segment elevation acute myocardial infarction. Factors with an OR>1 are associated with the use of thienopyridines. ACE-I indicates angiotensin-converting enzyme inhibitor; CAD, coronary artery disease; CABG, coronary artery bypass grafting; GP, glycoprotein; LMWH, low molecular weight heparin; PCI, percutaneous coronary intervention.
In-Hospital Major Bleeding
Major bleeding rates during hospitalization were lowest in patients who received fibrinolytic drugs only, and were highest in patients who received both fibrinolytic drugs and thienopyridines (2.3% vs 4.6%, respectively; P<.001) (Table 2). After adjusting for the GRACE variables for bleeding risk14 and PCI, the risk of major bleeding was higher in patients who received thienopyridines at some time during hospitalization who received either fibrinolytic drugs alone or neither therapy. While treatment with a thienopyridine was independently associated with an increased risk of (odds ratio [OR] = 1.68; 95% confidence interval [95% CI], 1.23-2.31) (Table 3).
In-hospital Mortality
Hospital death rates were lowest in patients receiving both fibrinolytic drugs and thienopyridines and highest in patients receiving neither fibrinolytic drugs nor thienopyridines (3.0% vs 15%, respectively) (Table 2). After adjusting for the potentially confounding variables included in the GRACE risk model for hospital mortality15 and PCI, the use of thienopyridines, both during the first 24 h and at any time during hospitalization, was associated with a significantly lower death rate (OR =0.50; 95% CI, 0.39-0.60) (Table 3).
DISCUSSION
The results of this large observational study in patients with STEMI showed that bleeding was more common in patients who received thienopyridines with or without fibrinolytic drugs than in patients bleeding, it was also associated with a lower risk of in-hospital death.
Increasing Use of Thienopyridines in STEMI
These data from the large, multinational GRACE study show a progressive increase in the use of thienopyridines in patients with STEMI, similar to that observed in other contemporary registries.16 The administration of thienopyridines, in particular clopidogrel, in association with aspirin appears to be effective in reducing the risk of ischaemic events in patients with non-ST elevation ACS,7 and in preventing the thrombotic occlusion of coronary arteries after PCI and stent implantation.8,17-19 Moreover, the early use of clopidogrel in patients with STEMI who receive fibrinolytic therapy is associated with a better outcome, as demonstrated in the Clopidogrel as an Adjunctive Reperfusion Therapy (CLARITY)9,20 and the Clopidogrel and Metoprolol in Myocardial Infarction (COMMIT)10 Randomized clinical trials.
The use of thienopyridines was more common in patients undergoing PCI (Table 1, Figure), but a significant number of patients who did not undergo invasive treatment received thienopyridines along with aspirin, unfractionated heparin, or low molecular weight heparin. Before the publication of the CLARITY9 and COMMIT10 results in March 2005, no clinical data were available to support the use of clopidogrel or ticlopidine in patients with STEMI; therefore the increase in use could be attributed to a perception of benefit not supported by the available clinical evidence at that time. In addition, patients receiving thienopyridines were more likely to receive glycoprotein IIb/IIIa inhibitors, statins, low molecular weight heparins, and angiotensin-converting enzyme inhibitors.
Bleeding Complications
Major bleeding was more common than that observed in randomized clinical trials, probably reflecting a more liberal use of antithrombotics without the protective inclusion and exclusion criteria and the more rigid control enforced in clinical trials. Treatment with thienopyridines was associated with an increased risk of major bleeding in both patients who received and who did not receive treatment with fibrinolytic drugs. The use of thienopyridines was also an independent predictor for major bleeding after correction for other factors related to an increase in bleeding complications in patients with STEMI. Because the mechanisms of action of aspirin, thienopyridines, glycoprotein IIb/IIIa inhibitors, fibrinolytic drugs, and heparins are different, their combined use may exert an additive antithrombotic effect but may also increase the potential risk of bleeding. An increase in bleeding complications has been reported with the combination of aspirin and clopidogrel,7 and a small and statistically inconclusive excess bleeding risk has been reported in patients referred for urgent surgery21 (note: surgical bleeding was excluded from the present analysis). However, the risk of bleeding complications does not appear to be increased significantly with triple antiaggregant therapy using aspirin, thienopyridines, and glycoprotein IIb/IIIa inhibitors,17,20,22 or with high clopidogrel doses associated with percutaneous coronary revascularization.23-25 No significant risk of bleeding was observed with the combined use of clopidogrel and fibrinolytics in the well selected groups of STEMI patients with low bleeding risk included in the CLARITY and COMMIT trials.9,10 The increase in bleeding risk in the present study could be explained by a more liberal use of antithrombotic drugs, without the careful clinical selection of patients with low bleeding risk observed in clinical trials.
Outcome
All-cause in-hospital mortality was lower in patients with STEMI who were treated with thienopyridines, especially in association with fibrinolytic therapy. In addition, the use of thienopyridines was associated with lower inhospital mortality after adjusting for a variety of factors previously shown to be associated with short-term mortality after an ACS. The lower mortality rate associated with thienopyridine use may reflect a beneficial effect of treatment but many confounding factors may also influence this association. The clinical effects of thienopyridines have been reported in patients with non-ST elevation ACS7 and in patients undergoing PCI,8,17-19,21-30 but the clinical benefit was only significant for combined end points and no direct benefit was reported in relation to mortality. In the CLARITY study, the addition of clopidogrel and aspirin to fibrinolytic therapy improved clinical outcome, but no difference in mortality was observed in patients who received clopidogrel or placebo.9 In the COMMIT trial, a modest but significant reduction in mortality was observed in patients receiving clopidogrel compared to placebo.10 It is difficult to explain the significant reduction in mortality found in the present study. It might be attributed to mechanisms that are difficult to ascertain in a non-randomized observational study or to an effect apparent only in higher-risk groups. Patients treated with thienopyridines were in general better treated, more frequently receiving medications that are highly recommended in practice guidelines (Table 1). It is interesting to note that patients included in the GRACE registry were at substantially higher risk than those enrolled in the CLARITY and COMMIT trials. In the GRACE registry, hospital mortality in patients who were not treated with thienopyridines or fibrinolytics was 15%, whereas 28-day mortality in patients who received neither drug was 10% in the COMMIT trial.10 Cardiovascular death at 30 days was only 4.5% in the placebo group of the CLARITY trial.9
Limitations and Strengths of the Study
While registry studies are always subject to potential biases, they are valuable for studying real-world practice patterns and detecting rare side-effects of drugs and drug interactions.31 The nature of GRACE, as well as any other registry with treatments not randomized in selected groups of patients, prevents clear conclusions being drawn regarding the benefit and potential risk of treatments. The findings may be an overestimation in view of the fact that it is impossible to control for all of the differences in baseline variables. Unmeasured variables may have led to clinicians deciding not to use thienopyridines, or indeed other pharmacological and interventional therapies in some high risk patients with complex comorbidity. Besides, the GRACE registry does not collect information on specific thienopyridine drugs, dosing and adherence to the treatment throughout the study period, preventing a more detailed analysis of the data. Furthermore, the 2 populations differ in terms of their baseline characteristics, with patients treated with a thienopyridine and no fibrinolytic having more important risk characteristics than those who received a fibrinolytic alone. Another important limitation is the lack of a standard definition of bleeding. The definition used in the GRACE study is similar to those used in other studies, but the rates of bleeding are not truly comparable with those associated with other definitions. Finally, it is impossible to assess a causal relationship between an endpoint and treatments from registry data. On the other hand, GRACE is a multicentre, prospective registry including unselected patients with ACS, and thus provides unbiased information on treatments and outcomes in clinical practice which may differ from those expected on the basis of results from randomized clinical trials involving selected populations of patients with well-defined inclusion and exclusion criteria. Thus, information obtained from large registries complements the information obtained in clinical trials, and is particularly relevant for exploring secondary effects in populations that are not protected by the rigid exclusion criteria of clinical trials. However, reliable conclusions cannot be established with respect to contradictions with the results of clinical trials, in particular in regard to the benefit of treatments.
CONCLUSIONS
The proportion of patients with STEMI who received thienopyridines has been progressively increasing, and over 70% of patients with STEMI now receive thienopyridines. Major bleeding was more common than in clinical trials, suggesting that less attention was given to the appropriate use of antithrombotics. Major bleeding was more common in patients who received thienopyridines, although the use of thienopyridines during the first 24 h post-STEMI was not found to be an independent risk factor for bleeding. A significantly lower death rate was also observed, suggesting that, overall, the use of thienopyridines may improve outcome in patients with STEMI.
ACKNOWLEDGMENTS
The authors thank all the physicians and nurses who are participating in GRACE. The GRACE study is supported by an unrestricted grant from Sanofi-Aventis to the Center for Outcomes Research, University of Massachusetts Medical School. We thank Dr Sophie Rushton-Smith for providing editorial support. To find out more about GRACE, visit the website at www. outcomes.org/grace.
SEE ARTICLE ON PAGES 474-8
ABBREVIATIONS
ACS: acute coronary syndrome
GRACE: Global Registry of Acute Coronary Events
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
GRACE is funded by an unrestricted educational grant from Sanofi-Aventis to the Center for Outcomes Research, Worcester, MA, USA. The authors have received grant support for GRACE and for this manuscript from Sanofi-Aventis.
Correspondence:
Dr. J. López-Sendón.
Departamento de Cardiología. Hospital Universitario La Paz. Paseo de la Castellana, 261. 28046 Madrid. España.
E-mail: jlsendon@terra.es
Received June 11, 2008.
Accepted for publication January 5, 2009