Distension of the ischemic region has been related to an increased incidence of spontaneous ventricular arrhythmias following coronary occlusion. This study analyzed whether regional ischemic distension predicts increased ventricular fibrillation inducibility after coronary occlusion in swine.
MethodsIn 18 anesthetized, open-chest pigs, the left anterior descending coronary artery was ligated for 60min. Myocardial segment length in the ischemic region was monitored by means of ultrasonic crystals. Programmed stimulation was applied at baseline and then continuously between 10 and 60min after coronary occlusion.
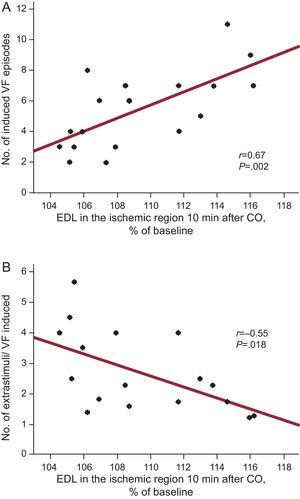
ResultsCoronary occlusion induced a rapid increase in end-diastolic length in the ischemic region, which reached 109.4% (0.9%) of baseline values 10min after occlusion (P<.001). On average, 6.6 (0.5) stimulation protocols were completed and 5.4 (0.6) ventricular fibrillation episodes induced between 10 and 60min of coronary occlusion. Neither baseline serum potassium levels nor the size of the ischemic region were significantly related to ventricular fibrillation inducibility. In contrast, the increase in end-diastolic length 10min after coronary occlusion was associated directly (r=0.67; P=.002) with the number of induced ventricular fibrillation episodes and inversely (r=–0.55; P=.018) with the number of extrastimuli needed for ventricular fibrillation induction.
ConclusionsRegional ischemic expansion predicts increased ventricular fibrillation inducibility following coronary occlusion. These results highlight the potential influence of mechanical factors, acting not only on the triggers but also on the substrate, in the genesis of malignant ventricular arrhythmias during acute ischemia.
Keywords
.
INTRODUCTIONMalignant ventricular arrhythmias in the setting of acute myocardial ischemia are a frequent cause of sudden death and represent a major public health burden.1–4 However, the underlying mechanisms of these arrhythmias are complex and not fully understood.1,2,5 In contrast, it is well known that stretching of myocardial tissue induces electrophysiological changes and is a trigger for arrhythmias.6–9 Since coronary occlusion rapidly increases the mechanical tension in the ischemic region and in the border zone,10–12 it is not surprising that the potential influence of mechanical changes on the genesis of ischemic ventricular arrhythmias has attracted research attention.
In this respect, an increased arrhythmogenicity has been described in the border zone,13,14 an area subjected to mechanical tension during regional ischemia, and also in association with spontaneous15–17 or induced13,18 left ventricular distension following coronary occlusion.
The results of all these studies support a role of mechanical factors in the genesis of ischemic malignant arrhythmias. However, it is unclear to which extent these factors might exert their potential influence by triggering premature ventricular beats initiating the arrhythmia, by increasing the vulnerability of the substrate, or by both mechanisms. To help clarify this issue, the present study aimed to assess whether distension of the ischemic region predicts ventricular fibrillation (VF) inducibility by programmed stimulation during regional ischemia in pigs.
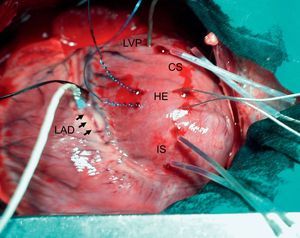
METHODSAnimal PreparationAfter approval by our Institutional Research Commission and in accordance with the recommendations of the American Physiological Society for laboratory research involving animals, 19 farm pigs of both sexes (40 [1]kg) were premedicated with 10mg/kg intramuscular azaperone and anesthetized with 30mg/kg intravenous thiopental sodium. Animals were intubated and mechanically ventilated (MA-1B ventilator, Bennett; Santa Monica, California, United States) with room air. Anesthesia was maintained with a continuous infusion of thiopental. One femoral artery and vein were percutaneously catheterized, a midline sternotomy was performed and the pericardium was opened. The left anterior descending coronary artery (LAD) was dissected at its mid segment and surrounded by an elastic snare. A 2-mm width Doppler flow probe (Transonic Systems; Ithaca, New York, United States) was placed adjacent to the snare. Two pairs of ultrasonic crystals 1mm in diameter were inserted into the inner third of the left ventricular wall. The crystals were placed approximately 1cm apart along a plane perpendicular to the long axis of the ventricle, 1 pair in the myocardium to be made ischemic and the other in the lateral wall. A micromanometer-tipped catheter (Mikro-tip, Millar; Houston, Texas, United States) was inserted into the left ventricle through a small incision in its lateral wall. Two hook electrodes were implanted 5-10mm apart in the myocardium at both sides of the predicted border zone. Figure 1 shows an example of the experimental preparation.
Experimental preparation. CS and IS, pairs of ultrasonic crystals inserted in the myocardium in the control and ischemic segment, respectively; HE, hook electrodes used for ventricular stimulation; LAD (and arrows), left anterior descending coronary artery, surrounded at its mid segment by a snare and a flow meter; LVP, micromanometer-tipped catheter for left ventricular pressure measurements.
Programmed bipolar stimulation was performed after hemodynamic stability was attained by means of a UHS 20 stimulator (Biotronik; Berlin, Germany) connected to the electrodes in the border zone. After a drive train of 8×600ms, up to 3 extrastimuli were applied with an initial 400ms S1-S2 interval, which was reduced at 10-ms steps until VF appeared or the effective refractory period was reached. In the latter case, the S1-S2 interval was set at 30ms above the effective refractory period and the second and third extrastimuli were applied according to the same routine. If VF occurred, the heart was defibrillated with 10-20J shocks. After this protocol was completed, the LAD was occluded and programmed stimulation was restarted 10min after coronary occlusion and continued for the whole ischemic period, allowing 3min recovery after each defibrillation. If no VF occurred, the interval until the next attempt was reduced to 2min.19 The 10-min interval before starting programmed stimulation during ischemia was chosen to ensure the best quality of segment length measurements, since it allowed for maximal ischemic end-diastolic expansion to occur10,11,15–17 and minimized the risk of displacement of the ultrasonic crystals by defibrillation shocks. One animal was excluded due to incessant VF immediately after coronary occlusion. Thus, this series was composed of 18 valid experiments.
Study MonitoringSerial arterial blood gases were obtained to adjust the ventilator parameters. Baseline serum potassium levels were measured. Aortic pressure was continuously monitored. The ultrasonic segment signals were analyzed with an ultrasonic dimension system (System 6/200, Triton Technology; San Diego, California, United States) and monitored with an oscilloscope (HM 205-3, Hameg; Frankfurt, Germany). These signals, along with lead II of the electrocardiogram, aortic pressure, LAD blood flow and left ventricular pressure and its first derivative (dP/dt) were continuously recorded, digitized (ML795 PowerLab System, AD Instruments; Mountain View, California, United States) and stored on the hard disk.
The number of ectopic ventricular beats throughout the ischemic period was counted and the occurrence of spontaneous VF recorded, as well as the total number of induced VF episodes and the mean number of extrastimuli applied for VF induction. This latter variable was defined as the sum of extrastimuli (1-3 per protocol) applied in all the stimulation protocols administered to an animal during ischemia divided by the number of induced VF episodes in this period.
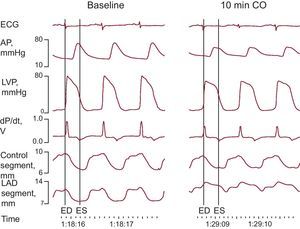
Segment Length Measurements and Postmortem StudiesSegment length measurements were performed on the digital records as previously described.11,15–17 End-diastole was defined as the beginning point of the rapid upslope of the dP/dt tracing, and end-systole was defined as the point of minimum dP/dt (Fig. 2). End-diastolic and end-systolic segment lengths (EDL, ESL)—that is, the distance between the two ultrasonic crystals of each pair at end-diastole and end-systole, respectively—and maximal segment length were measured at baseline and at 5, 10, 15, 30, 45, and 60min after coronary occlusion. Systolic shortening was calculated as follows (%): (EDL–ESL)×100/EDL. EDL was expressed as a percentage of values before coronary occlusion. Systolic bulging during the ischemic period was calculated as follows (%): (maximal segment length during systole–EDL)×100/EDL.
Example of electrocardiogram, hemodynamic, and segment length monitoring at baseline and 10min following coronary occlusion. AP, arterial pressure; CO, coronary occlusion; dP/dt, first derivative of LVP; ECG, electrocardiogram; ED, end-diastole; ES, end-systole; LAD, left anterior descending coronary artery; LVP, left ventricular pressure.
Sixty minutes after coronary occlusion, 5mL of 10% fluorescein were injected into the left atrium and the heart was excised and cut in slices, which were weighed and photographed under ultraviolet light. The area at risk was measured semiautomatically in the digitized images using commercially available software (Microimage 3.0, Olympus Optical; Hamburg, Germany) and the mass of the ischemic region was calculated from these measurements and the weight of the slices.11,15–17
Statistical AnalysisThe statistical analysis was performed using SPSS software. Data are expressed as means (standard errors). Paired Student t-tests or analysis of variance for repeated measures was used to assess changes in physiological variables throughout the experiment. The association between EDL and VF inducibility was assessed by simple regression analysis. P values <.05 were considered significant.
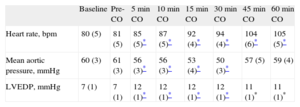
RESULTSHemodynamic DataHemodynamic data are summarized in Table 1. Baseline parameters were within the normal range and were not modified by ventricular stimulation before ischemia (P=NS). Heart rate increased after coronary occlusion (P=.03) and augmented progressively during the ischemic period (P=.001). Mean aortic pressure decreased slightly after LAD ligation (P=.003) and experienced subsequent minor variations (P=.04). Left ventricular end-diastolic pressure increased after coronary occlusion (P<.001) and remained stable thereafter (P=NS). LAD flow at baseline was 12 (1)mL/min.
Hemodynamic Data.
| Baseline | Pre-CO | 5min CO | 10min CO | 15min CO | 30min CO | 45min CO | 60min CO | |
| Heart rate, bpm | 80 (5) | 81 (5) | 85 (5)* | 87 (5)* | 92 (4)* | 94 (4)* | 104 (6)* | 105 (5)* |
| Mean aortic pressure, mmHg | 60 (3) | 61 (3) | 56 (3)* | 56 (3)* | 53 (4)* | 50 (3)* | 57 (5) | 59 (4) |
| LVEDP, mmHg | 7 (1) | 7 (1) | 12 (1)* | 12 (1)* | 12 (1)* | 12 (1)* | 11 (1)* | 11 (1)* |
CO, coronary occlusion; LVEDP, left ventricular end-diastolic pressure.
Data are means (standard error).
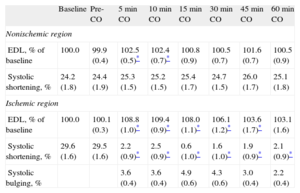
At baseline, absolute EDL averaged 11.5 (0.5)mm in the control zone and 13.4 (0.8)mm in the LAD region. Segment length measurements are summarized in Table 2. EDL and systolic shortening were not modified by ventricular stimulation before ischemia (P=NS). In the control zone, EDL showed minor, though significant (P=.02), variations throughout the experiment, whereas systolic shortening remained constant (P=NS). In the LAD region, EDL underwent significant changes (P<.001) consisting of a rapid increase (Fig. 2) after coronary ligation (which at 10min averaged 109.4% [0.9%] of the preischemic value; P<.001) with some attenuation of this increase at the end of the experiment. Coronary occlusion also immediately abolished systolic shortening (P<.001) and prompted the occurrence of systolic bulging in the LAD region.
Segment Length Measurements.
| Baseline | Pre-CO | 5min CO | 10min CO | 15min CO | 30min CO | 45min CO | 60min CO | |
| Nonischemic region | ||||||||
| EDL, % of baseline | 100.0 | 99.9 (0.4) | 102.5 (0.5)* | 102.4 (0.7)* | 100.8 (0.9) | 100.5 (0.7) | 101.6 (0.7) | 100.5 (0.9) |
| Systolic shortening, % | 24.2 (1.8) | 24.4 (1.9) | 25.3 (1.5) | 25.2 (1.5) | 25.4 (1.7) | 24.7 (1.5) | 26.0 (1.7) | 25.1 (1.8) |
| Ischemic region | ||||||||
| EDL, % of baseline | 100.0 | 100.1 (0.3) | 108.8 (1.0)* | 109.4 (0.9)* | 108.0 (1.1)* | 106.1 (1.2)* | 103.6 (1.7)* | 103.1 (1.6) |
| Systolic shortening, % | 29.6 (1.6) | 29.5 (1.6) | 2.2 (0.9)* | 2.5 (0.9)* | 0.6 (1.0)* | 1.6 (1.0)* | 1.9 (0.9)* | 2.1 (0.9)* |
| Systolic bulging, % | 3.6 (0.4) | 3.6 (0.4) | 4.9 (0.6) | 4.3 (0.6) | 3.0 (0.4) | 2.2 (0.4) | ||
CO, coronary occlusion; EDL, end-diastolic segment length.
Data are means (standard error).
Baseline serum potassium averaged 3.18 (0.12)mmol/L and the mean area at risk represented 13.2% (0.3%) of ventricular mass.
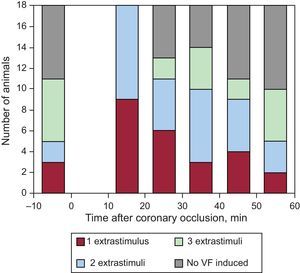
Ventricular Fibrillation Inducibility and its Association With the Distension of the Area at RiskAnimals had 100 (15) premature ventricular beats during ischemia (11 [6] in the first 10min and 90 [16] between 10 and 60min after coronary occlusion). Before coronary occlusion, VF was induced by programmed stimulation in 11 animals (61%), although 2 or 3 extrastimuli were often needed to induce the arrhythmia (Fig. 3). Between 10 and 60min after coronary occlusion, a total of 6.6 (0.5) stimulation protocols per animal were completed that resulted in VF induction in all animals with an average of 5.4 (0.6) VF episodes per animal. In addition, 12 animals (67%) had 1.3 (0.5) episodes of spontaneous VF—not induced by programmed stimulation—during the occlusion period, the first episode in each animal occurring 26 (4)min (range 13-46min) after coronary occlusion.
As Figure 3 also shows, the distribution of induced VF episodes throughout the ischemic period was not homogeneous. Maximal vulnerability was observed between 10 and 20min after coronary occlusion, after which time the frequency of the episodes tended to decline and the number of extrastimuli needed to induce the arrhythmia tended to augment.
Vulnerability to VF was not significantly associated with baseline potassium levels (r=–0.22; P=NS) or with the size of the area at risk (r=0.30; P=NS). In contrast, it was related to the magnitude of the distension of the ischemic region early after coronary occlusion. The increase in EDL in the ischemic region 10min after coronary occlusion was associated directly (r=0.67; P=.002) with the number of induced VF episodes and inversely (r=–0.55; P=.018) with the number of extrastimuli needed for VF induction (Fig. 4). Neither systolic shortening nor systolic bulging at the same time point was related to VF inducibility.
Association between end-diastolic segment length (expressed as a percentage of baseline value) measured 10min after coronary occlusion and the number of ventricular fibrillation episodes induced (A) or the total number of extrastimuli (including those applied in protocols not resulting in ventricular fibrillation induction) needed for the ventricular fibrillation episode induced (B) between 10 and 60min of coronary occlusion. CO, coronary occlusion; EDL, end-diastolic segment length; VF, ventricular fibrillation.
The increase in EDL was not associated with the number of premature ventricular beats during the whole ischemic period (r=–0.23; P=NS) or with those occurring during the first 10min (r=0.11; P=NS) or between 10 and 60min after coronary occlusion (r=–0.26; P=NS). With regard to spontaneous VF, it was not related to baseline hemodynamic variables or potassium levels, but was associated with the size of the area at risk (14.8% [7.1%] vs 7.9% [0.9%] of ventricular mass in animals with and without spontaneous VF, respectively; P=.013).
DISCUSSIONThis study shows that distension of the ischemic region early after coronary occlusion is associated with an increased susceptibility to VF by programmed stimulation in swine. The results highlight the potential role of mechanical factors in ischemic VF and suggest a direct contribution of these factors to the substrate of this arrhythmia independent of any other possible influence on the triggers.
Mechanical Influences on Sustained and Non-sustained Ventricular Arrhythmias During Acute Regional IschemiaAlthough the arrhythmogenic effect of myocardial stretch and ventricular distension has long been demonstrated in different experimental preparations,6–8 information in the setting of acute regional ischemia is scarcer. Earlier studies described changes in action potential duration induced by increasing the loading conditions.18 In previous studies in our laboratory, the magnitude of acute distension of the ischemic region was consistently associated with subsequent occurrence of spontaneous VF following coronary occlusion in anesthetized pigs.15–17 Coronel et al.13 reported that, in isolated, perfused swine hearts subjected to coronary occlusion, both the frequency and severity of spontaneous ventricular arrhythmias were increased if intraventricular pressure was raised with a balloon. Finally, mapping of those hearts13 and modeling studies of rabbit ventricles14 have identified the border zone as a frequent origin of ventricular premature beats during regional ischemia.
Stretch may increase ventricular arrhythmogenicity following coronary occlusion by triggering premature beats, by increasing myocardial vulnerability to sustained arrhythmias, or by both mechanisms. However, and perhaps due to heterogeneous methodologies, those previous studies have differed in the relative contribution attributed to each of these mechanisms. Concurring with the present results, in none of our previous studies was the early increase in EDL after coronary occlusion correlated with the number of premature ventricular beats during the Ib phase of arrhythmias, despite showing a strong association with spontaneous VF.15–17 In the study by Coronel et al.,13 augmenting ventricular workload facilitated the occurrence of both premature beats and malignant arrhythmias, although the increase appeared to be relatively higher for the latter. Finally, the modeling study by Jie et al.,14 indicated that mechanical activity influences both the triggers and the substrate of ischemic arrhythmias in the rabbit ventricle. Whatever may be the influence of regional distension on triggering premature ventricular beats after coronary occlusion, its association with VF inducibility observed herein strongly suggests an independent effect on the substrate of this arrhythmia.
Several mechanisms might account for this potential influence. First, it could be mediated in part by stretch-activated channels.20,21 In this respect, previous studies in this animal model in our laboratory failed to reduce the number of premature beats or the incidence of sustained arrhythmias (although they were not powered for this latter purpose) with intravenous16 or intracoronary17 administration of gadolinium, a stretch-activated channel blocker.20 However, those studies were limited by the difficulty of ensuring an adequate delivery of the drug to the border zone. Second, mechanical distension may trigger autonomic reflexes increasing sympathetic activity,22,23 which, by itself, reduces the fibrillatory threshold.24,25 Finally, it might act through alternative mechanisms such as beta-adrenergic receptor activation26 or by affecting cytosolic calcium concentration or its effect on contractile proteins.27,28
Comparison With Previous StudiesAn increased inducibility of VF during ischemia has previously been reported in different species and experimental preparations.19,29–31 In a study particularly relevant to ours due to similarities in animal species and stimulation protocol, De Groot et al.19 observed that maximal inducibility of VF after coronary occlusion in isolated, blood-perfused swine hearts coincided temporally with the rise in tissue resistivity indicative of cellular uncoupling. At variance with these results, in our study maximal vulnerability was observed earlier, at between 10 and 20min of ischemia, a time interval mostly prior to the expected occurrence of cellular uncoupling in this animal model.32–34 The frequent occurrence of spontaneous VF in subsequent time intervals, which interfered with programmed stimulation and reduced the total number of protocols that could be completed, might help explain this apparent discrepancy. Hypothetically, the attenuation of the increase in EDL observed in later stages of coronary occlusion (which, perhaps in relation to the intervention applied, was more apparent in the present study than in previous studies in the same model10,11,15–17,34) might also have contributed to these results.
Methodological Considerations and LimitationsIn the present study, serum potassium levels and the size of the area at risk, 2 well-known predictors of VF following coronary occlusion1 (and the latter being associated with spontaneous VF in this study) showed no significant association with VF inducibility. This is possibly due in part to the homogeneity in the animals and experimental conditions and concurs with previous results observed for spontaneous VF in the same model.15–17 The lack of serial measurements of potassium levels throughout the experiment constitutes a limitation, although previous studies in this model have shown only minimal variations in potassium levels following coronary occlusion.34 The minor, although significant, reduction throughout the experiment of the mild increase in EDL observed in remote myocardium immediately after coronary occlusion also concurs with previous observations in the same model.35
For the analysis of the association between EDL and arrhythmia inducibility, a single time point of EDL was taken into account. Considering multiple measurements of EDL throughout the ischemic period would have been preferable but would have complicated significantly the analysis and interpretation of the results. In addition, once continuous stimulation started, the occurrence of multiple episodes of VF with subsequent defibrillation shocks sometimes hampered monitoring of EDL changes. However, the stability of EDL in the ischemic region observed in previous studies10,11,16,17,34 is against a significant dispersion of this variable with consequences on arrhythmogenicity in the present study. The absence of a control group without coronary occlusion, the fact that we did not measure directly mechanical stress at the ischemic border and the intrinsic susceptibility of the animals to VF—exemplified by the large proportion in which this arrhythmia was induced before coronary occlusion—represent additional limitations.
Due to its observational nature, our study cannot establish a cause-effect relationship of the association between regional ventricular distension and VF inducibility. However, a real influence of these mechanical changes on susceptibility to VF is supported by the fact that this association concurs with a higher frequency of spontaneous VF associated with regional distension in the same model15–17 and with other studies using different approaches.13,14
CONCLUSIONSThe results of the present study suggest that mechanical factors enhance myocardial vulnerability to malignant ventricular arrhythmias following coronary occlusion. The potential mechanisms of this association are worthy of investigation, given the high prevalence and lethality of these arrhythmias in patients with acute coronary syndromes.
FUNDINGThis work was supported by the Spanish Ministry of Health (FIS PI051745 and SAF 2008-3067), the Spanish Society of Cardiology (Beca 2006 para Investigación Básica) and Redes Temáticas de Investigación Cardiovascular (RECAVA).
CONFLICTS OF INTERESTNone declared.