Keywords
INTRODUCTION
According to the oxidation hypothesis of atherogenesis, increased generation of free radicals and reactive oxygen species, possibly associated with weakened antioxidant defense mechanisms, is responsible for atherosclerosis.1 Oxidized low-density lipoproteins (LDL) and derivative products generated in association with these oxidative processes can accumulate in the arterial wall and accelerate the atherosclerotic process.1,2 In principle, any enzymatic system that generates free radicals could be implicated in the oxidation of LDL particles, for example, the NADPH oxidase system, the myeloperoxidase system, the P450 system, the mitochondrial electron transport chain, the xanthine oxidase system, and the lipoxygenase system. The importance of lipoxygenase was first investigated in studies carried out in vitro,3-5 although several in vivo studies have since corroborated the in vitro findings.6-10 The role of lipoxygenase has been further confirmed by experiments with apolipoprotein E and 12/15-lipooxygenase double knockout (apoE--//L-12LO-/-) mice, in which atherosclerotic lesions decreased substantially,11,12 although contradictory findings have been reported.13
Seeding Mechanism
The action of 15- and 12-lipoxygenases on arachidonic and linoleic acid generates mainly hydroperoxyoctadecadienoic acid (HPODE) and hydroperoxyeicosatetraenoic acid (HPETE). Several experimental findings support, at least partially, a mechanism of formation of mildly oxidized LDL whereby phospholipids derived from arachidonic acid are oxidized and these metabolic products of lipoxygenases, particularly HPODE and HPETE, are taken up by different LDL lipids.14 However, LDL particles also need to be seeded with reactive oxygen species for extensive oxidation to occur.14,15 High-density lipoproteins (HDL) and their main structural component, apolipoprotein A1, can prevent oxidation of LDL.1 A number of molecular mechanisms may arise after transfer of cholesterol ester hydroperoxides (CEOOH) from LDL to HDL, in a process partially determined by the enzymatic activity of the cholesterol ester transfer protein (CETP).16 Nevertheless, the role of CETP is potentially atherogenic and CETP inhibitors (JTT-705 and torcetrapib) have been shown to afford protection in several clinical trials.17,18 It should, however, be remembered that the mechanism of cholesterol ester transfer from HDL to apolipoprotein B lipoproteins (in a process that leads to the exchange of triglycerides), operates both ways and depends on the concentration of triglyceride-rich lipoproteins.16,19 The renovation of HDL rather than a high concentration of HDL is thought to be most relevant to vascular protection. In particular, the action of CETP in HDL facilitates esterification by lecithin-cholesterol acyltransferase (LCAT) and therefore, the efflux of cholesterol. In turn, the combined action of CETP and hepatic lipase on mature HDL (HDL2) generates HDL fractions that contain lower concentrations of lipids and that are better cholesterol acceptors.17,20
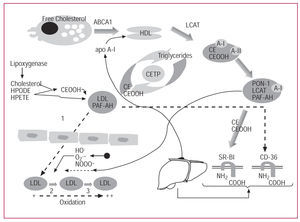
The mechanism of formation of mildly oxidized LDL comprises at least 3 steps.14,15 The first consists of the aforementioned seeding of LDL particles with the metabolic products of arachidonic acid and with CEOOH.14 In the second step, LDL crosses the subendothelial space, where there is an additional accumulation of reactive oxygen species. The third step involves oxidation of LDL phospholipids on reaching a threshold concentration of reactive oxygen species.15 The enzymatic activity of paraoxonase presumably intervenes in the first of these steps. The mechanisms covered by this review are represented schematically in Figure 1.
Figure 1. Schematic representation of high-density lipoprotein (HDL) metabolism and its protective role against oxidation of low-density lipoproteins (LDL).
Measuring Oxidized Phospholipids and Hydroperoxides in Plasma
The method used for measuring lipid hydroperoxides in plasma depends on whether total concentrations or concentrations in subfractions are determined. The wide variety of techniques used has led to discrepancies in the measurement of the normal content of peroxides in healthy subjects. Bowry et al,21 using high performance liquid chromatography with chemoluminescence detection, found that HDL in both plasma and isolated lipoproteins (HDL and LDL) carried 85% of the total content of CEOOH and phospholipid hydroperoxides, detected as hydroperoxides of phosphatidylcholine. In contrast, Nourooz-Zadeh et al22 analyzed the total content of lipid hydroperoxides and found that they resided mainly in LDL particles and not in HDL ones.
Paraoxonases 1, 2, and 3 (PON-1, PON-2, and PON-3)
Human paraoxonase/arylesterase (PON-1) (EC 3.1.1.2) is a calcium-dependent glycoprotein that is present bound to HDL particles. Investigators have attempted to demonstrate that serum paraoxonase decreases the risk of coronary artery disease by destroying proinflammatory molecules involved in the initiation and progression of atherosclerotic lesions.23 The antiatherogenic potential of paraoxonase is derived from its capacity to hydrolyze oxidized lipids, phospholipids, and CEOOH, thus preventing them from accumulating in LDL particles. In vitro studies have shown that paraoxonase activity prevents oxidation of LDL particles24 and even oxidation of the HDL particles themselves.25 PON-1 can act in a similar fashion in vivo.26-28 PON-1 is also characterized by hydrolyzing different carboxylic acid esters and some organophosphates. Although the actual physiological substrate of the PON-1 enzyme is not well known, it has been possible to determine its serum activity using paraoxon as a substrate. Determination of this activity has shown that serum levels of paraoxonase vary from individual to individual but remain relatively constant in a given individual.23 A range of physiopathological situations linked to increased oxidative stress and environmental factors can lower serum paraoxonase activity.29-34 Moreover, increased oxidative stress has been reported in PON1 knockout mice and in apo E/PON1 double-knockout mice--models in which a different response to macrophage expression of PON2 and PON3 was reported.35,36
Binding between paraoxonase and HDL particles may explain the inverse relationship between HDL levels and coronary artery disease reported in a number of population studies.23 The antioxidant activity could be responsible for the protective role of paraoxonase. Such activity would be conserved even in the process of reverse cholesterol transport. Thus, esterification of excess cholesterol occurs on the surface of the HDL particles and is mediated by LACT activity, which is particularly sensitive to lipid hydroperoxides.37,38 It is important to remember that the enzymatic activity of paraoxonase is limited to certain subfractions of HLD39 and that apolipoprotein A1 is required to stabilize the enzyme.40
The human gene PON1 maps to the long arm of chromosome 7 (7q21-q22)41 and exhibits interesting polymorphims.42 The Gln192 Arg (Q/R) polymorphism is responsible for the PON1 A (Q) and PON1 B (R) alleles, which are associated with different levels of enzymatic activity according to the substrate. The Met55 Leu polymorphism is responsible for the appearance of the L (55 leucine) and M (55 methionine) alleles. The Met55 Leu variant is the one that modifies serum concentrations, but not the enzymatic activity of paraoxonase.43
The Gln192 Arg polymorphism of the PON1 gene has been associated with vascular disease in diabetic subjects44-47 and nondiabetic subjects.46 However, not all groups have found such an association.48-50 Some meta-analyses have been published that show a weak association between this variant and cardiovascular disease.34,51,52 Although other nongenetic determinants are partly responsible for interindividual variability of PON-1 activity, some authors think it appropriate to consider genotypes and PON-1 activity together in studies of association.34,53 Moreover, the fact that the association does not appear in all populations studied suggests that the variant does not correspond to a functional mutation but is rather a marker of another mutation in the PON1 gene itself or another nearby gene.54 There may also be interactions between the genotype and environment that have yet to be well characterized or that have a low prevalence in certain populations but that would be important for these studies.55,56
The possibility that the Met55 Leu polymorphism is a genetic risk factor for cardiovascular disease was assessed by Garin et al,43 who found that Leu55 homozygosis was an independent risk factor for cardiovascular disease in subjects with diabetes.
The mechanism by which PON1 polymorphisms increase susceptibility to cardiovascular disease is not known. The presence of the R192 and L55 alleles suggests greater paraoxonase activity towards paraoxon, although these alleles are variants associated with risk in certain populations. This posed an important dilemma for sometime because hydrolysis activity against paraoxon was linked to activity against the real enzyme substrate. Certain theories were put forward to explain this paradox. Reduced paraoxonase enzymatic activity after myocardial infarction,29 in familial hypercholesterolemia,30 in diabetes,31 and in association with renal failure32,33 suggested a direct influence of oxidative stress on the modulation of activity and enzyme concentration (Figure 2). On the other hand, greater hydrolytic activity against paraoxon or other exogenous substrates would not necessarily imply, as had been suggested, a greater antioxidative capacity. This latter insight contributed to the corresponding experimental confirmation in humans. Mackness et al57 found that the capacity of HDL particles to protect against LDL oxidation was greater for the QQ/MM homozygotes than for the RR/LL homozygotes (Figure 3). Cao et al58 observed that the differences in paraoxon hydrolysis resulting from the Gln192 Arg variant did not affect the capacity of the PON-1 protein to protect against oxidation of LDL particles. Aviram et al25 observed that deactivating the (calcium-dependent) PON-1 arylesterase activity did not suppress the capacity of the enzyme to inhibit LDL oxidation. In contrast, inhibitory activity could be suppressed by heating. These authors suggested that different active sites of PON-1, one corresponding to calcium-dependent paraoxonase activity, the other to calcium-independent activity, were responsible for protection against LDL oxidation.58
Figure 2. A. Significant reduction in paraoxonase activity in 68 diabetic men versus 93 nondiabetic men adjusted for age. B. Significant reduction in paraoxonase activity in 161 men after an acute coronary event and versus 184 age-adjusted controls.123 The bars represent mean and standard deviation. The comparison was made with the Student t test.
Figure 3. A. Differing protection against oxidation of L-1-palmitoyl-2-arachidonyl- sn-glycero-3-phosphorylcholine and hydroperoxyoctadecadienoic acid afforded by high-density lipoproteins (HDL) according to different isoforms of paraoxonase. The HDL samples were isolated by precipitation and adjusted with saline buffer to 20 units of arylesterase.107,108 The experiments were done by free cell assay (J Lipid Res. 2001;42:1308-17). B. Differing behavior of HDL with respect to preventing their own oxidation according to different paraoxonase isoforms. Determination of lipid peroxidation was done by the Xylenol Orange method (FOX) (Biochem J. 1996;313:781-6). All experiments were done in the presence of phenylmethylsulfonyl fluoride (PMSF).107,108
After the initial characterization of the PON1 gene, new PON-like genes, PON2 and PON3, were identified, also mapping to 7q21.3.59,60 Unlike PON1, which is mainly expressed in the liver, PON2 is expressed in a variety of tissues.
Mochizuki et al59 identified several forms of mRNA from the PON2 gene produced by alternative splicing or by use of a second transcription start site. Likewise, these authors characterized 2 polymorphisms in the gene coding sequence: Arg148 Gly and Cys311 Ser.
Sanghera et al61 observed that PON1 (Gln192 Arg) and PON2 (Cys311 Ser) polymorphisms both contributed synergistically to cardiovascular risk in Asian Indians.
As discussed earlier, the paraoxonase gene family comprises 3 members: PON1, PON2, and PON3. The physiological role of the corresponding gene products is of increasing interest. So far, the serum paraoxonase/arylesterase PON-1 and the paraoxonase PON-3 from rabbit serum have been characterized.62 Unlike PON-1, PON-3 presents only limited arylesterase activity and complete lack of paraoxonase activity; however, it rapidly hydrolyzes lactones and its protective activity against Cu2+--induced oxidation of LDL particles is greater than that reported for the PON-1 protein.
Which Enzyme Is Mostly Responsible for the Antioxidant Activity of HDL Particles?
The paraoxonase enzyme is not the only one that affords HDL particles protection against oxidation; other enzymes are implicated. The most important of these is platelet-activating factor acetylhydrolase (PAF-AH). Plasma PAF-AH enzymatic activity is responsible for deactivation of platelet-activating factor (PAF), thereby regulating its function and pathophysiological effects.63 Approximately 70% of the plasma activity of PAF-AH is associated with LDL particles and the rests with HDL particles, suggesting an active exchange between the 2 fractions.64 Lipid peroxidases are hydrolyzed by PAF-AH, which acts like phospholipase A2 but not like phospholipase C or D, and its antiatherogenic role is strongly debated.65-68 Marathe et al69 published an excellent study that suggested that PAF-AH and not the enzymatic activity of paraoxonase is responsible for all hydrolase activity of oxidized phospholipids. As discussed earlier, PON-1 is a calcium-dependent enzyme, whereas PAF-AH is not calcium dependent. PAF-AH is a phospholipase A2 that belongs to the serine-esterase family and, as such, its activity can be inhibited by specific inhibitors that block PAF-AH activity but not PON activity. In fact, paraoxonase lacks serine residues at its active site. With this strategy, it has been shown that PAF-AH is the only HDL phospholipase A2 and that PON-1 lacks phospholipase activity against PAF or oxidized phospholipids. Nevertheless, direct evidence suggests that PON-1 should at least be present for antioxidant and antiatherogenic effects to occur.27,70 Some authors have confirmed the PAF hydrolytic activity of serum PON-1 by using specific inhibitors of PAF-AH activity.71 In addition, in the knockout mouse model for the PON1 gene, PAF-AH activity is similar to that of the wild type.27,72 The HDL lipoprotein isolated from PON1 knockout mice is proinflammatory, that is, in absence of PON-1, PAF-AH activity is unable to maintain the antioxidant properties of the HDL particles.27,72 In transgenic animals that express human PON1, a certain degree of protection has been found against the development of atherosclerosis.27,70 Such protection has also been found in murine models that overexpress PAF-AH.67,73,74 Alternatively, coordinated action of both enzymes has been suggested in vivo, as their affinity for oxidized phospholipids varies according to the length of the esterified fatty acid chain at the sn-2 position.25,64,71,75,76 These excellent studies complete those done by Aviram et al77,78 and Rozenberg et al,36,79 who observed differential hydrolysis of oxidized lipids both in vitro and in atherosclerotic lesions using Q and R recombinant isoforms. Thus, the Q isoform lowered Cu2+--induced oxidation of the LDL particles by 33% compared to 20% for the R isoform. These latter studies focussed on developing methods to provide increasingly pure paraoxonase proteins, and so managed to show that the protein by itself seems unable to prevent oxidation of LDL particles.80-82 Recently, studies have been published of other molecular mechanisms that involve the interaction between HDL and paraoxonase and so may mediate vascular protection.83,84
Thiolactone Hydrolase Activity
Homocysteine (Hcy) measured as total plasma homocystine (tHcy) is considered as an independent and graded risk factor for cardiovascular disease.85-87 The determinants of changes in tHcy plasma concentration are by-and-large known and include both environmental and genetic factors.88-90 The molecular hypotheses that link elevated Hcy concentrations with the disease include direct toxicity towards endothelial cells, oxidation of Hcy, smooth muscle cell growth, and activation of genes important in the development of atherosclerosis.91-94 Elevated Hcy in plasma is associated with other processes related to proteins. Between 80% and 90% of plasma Hcy is bound to proteins, approximately half this protein bindings occurs through disulfide bridges and the other half through more stable amide bonds.95,96 Edition of Hcy by certain aminoacyl-t-RNA synthases inside the cell leads to the formation of the thioester homocysteine thiolactone.97 In vitro studies have shown that the formation of this lactone is directly proportional to the concentration of Hcy and inversely proportional to the concentration of methionine, and that this formation is inhibited by administration of folic acid.97,98 If most Hcy is bound into proteins and there is also mechanism that can edit Hcy, the next question would be whether the incorporation of Hcy to proteins happens during or after translation. Evidence from in vitro studies done with certain cell types shows that this incorporation occurs after translation.99,100 This is particularly important because in vitro studies have shown that N-homocysteinylation of proteins may be mediated by metabolic conversion of Hcy into its corresponding lactone in certain physiological situations. Detoxification of homocysteine thiolactone therefore constitutes a crucial mechanism. Different studies have shown hydrolysis of these lactones is one of the functions of the paraoxonase enzyme.99 The molecular identity has also been revealed. Is this hydrolytic activity against homocysteine thiolactone the main role of PON-1?
Thiolactone Hydrolase Activity and Paraoxonase Isoforms
Jakubowski et al101 showed that the hydrolysis activity of homocysteine thiolactone differs according to PON1 genotype. High thiolactonase activity was found in carriers of the R192 and L55 alleles, whereas activity was lower in carriers of the Q192 and M55 alleles. The authors suggest that low lactonase activity could be an important cardiovascular risk factor in subjects with high plasma concentrations of Hcy, an explanation supported by some studies. According to Billecke et al,102 the Q and R isoforms show variable specificity towards different lactones and also towards different carboxylic acid esters. This would certainly explain previous clinical observations on the differential pharmacological effects of lipid-lowering drugs on serum paraoxonase activity103-108 (Figure 4).
Figure 4. A: Mean concentration of total plasma homocysteine (tHcy) according to the PON1 genotypes Gln192 Arg RR (Arg/Arg); QR (Gln/Arg); QQ (Gln/Gln) in a control group of 243 healthy men not receiving pharmacological treatment.107 ,108 B: Mean concentration of total plasma homocysteine according to the PON1 genotype Gln192 Arg in a group of 20 patients treated with statins.107,108
The SR-B1 (CLA1) Gene
The metabolism of the HDL particles involves a selective cell uptake process such that HDL components enter the cholesterol ester but not the protein fraction.109 This selective uptake process is mediated in mouse by the scavenger receptor class B type 1 or SR-B1.110,111 This is the first HDL receptor that has been well characterized at the molecular level.110
In 1993, Calvo and Vega112 identified a new sequence related to the CD36 receptor and to the lysosomal integral membrane protein II (LIMPII). The new gene was denominated CLA1 (CD36 and LIMPII analogue 1). Murao et al113 confirmed that the SR-B1 sequence was 81% identical with the CLA1 sequence. Alternative splicing of the CLA1 gene produces 2 forms, giving rise to 2 messengers from which 2 proteins of 409 and 509 amino acids have been deduced. The form identified by Murao et al,113 similar to SR-B1, corresponds to the 509-amino-acid protein.
Acton et al110 have identified several polymorphisms of the sequence of the human CLA1 gene in a healthy control population. The authors characterized intron variants (introns 3 and 5) and exon variants (exons 1, 8 and 11) by single-chain conformational analysis and sequencing. Two findings are, in our opinion, particularly interesting. The Gly→Ser substitution located in the second coded amino acid in the cDNA molecule (G/A substitution in the first triplet base) has already been associated with higher plasma concentrations of HDL and lower concentrations of LDL in men. On the other hand, the C→T substitution, located in the third base of codon 350 of the cDNA molecule, is associated with lower concentrations of LDL in women.
CLA1 (SR-B1) and Antioxidant Properties of HDL
There are several mechanisms responsible for the antiatherogenic effects of HDL particles. The most important is reverse cholesterol transport, a mechanism by which HDL particles take up excess cholesterol from extrahepatic tissues. Cholesterol is esterified through LCAT action then selectively transported to the liver, where CLA-1 mediated entry into the cholesterol ester occurs110-112,114 (Figure 1). Uptake of cholesterol ester by the liver is coupled with the synthesis and secretion of bile acids.115,116 The importance of the antiatherogenic effects of CLA-1 lies in the metabolism of HDL, as shown by experiments with SR-B1 and LCAT knockout murine models and models, also in mouse, with transient overexpression of SR-B1.114,117-119
The CLA1/SR-B1 gene mediates the 2-way transport of cholesterol and nonesterified phospholipids between the HDL particles and different cell types. The physiological role of this 2-way mechanism has not been satisfactorily clarified.120
The antiatherogenic importance of HDL particles is also derived from their direct or indirect antioxidant activity, as they are able to sequester CEOOH from LDL and subsequently eliminate it through reverse transport as cholesteryl ester hydroxides (CEOH).121 Some authors have shown that most of the CEOOH in humans are associated with HDL,21 whereas others have found them to be associated with LDL particles.22 For the purposes of this review, it is important to highlight that the selective uptake of CEOOH by parenchymal hepatic cells, mediated by CLA-1, is approximately 3 times greater than for native cholesterol ester.121
The role of SR-B1 in the metabolism of HDL particles poses a fundamental question. It is necessary to determine whether hepatic expression of SR-B1 favors atherogenesis when HDL concentrations are reduced, or whether antiatherogenic actions occurs on elimination of cholesterol ester. The findings presented earlier reveal a fundamental antiatherogenic role. This is because expression occurs mainly in the liver, where its activity as a scavenger receptor would be important. Furthermore, regulation appears different, at least in macrophages and in Kupffer cells. Some regulation mechanisms are subtle. Thus, HDL particles themselves regulate the expression of SR-B1 in macrophages. Particles of HDL induce activation of PPAR-gamma (messenger and protein) and its translocation to the nucleus, but they also induce phosphorylation of PPAR-gamma mediated by MAP-kinases, so preventing expression of the response genes (SR-B1 and CD36 among others) and thereby suggesting a mechanism iny which HDL particles inhibit lipid accumulation.122
CLA-1 (SR-B1), PON-1, and Lipid Oxidation: Hypothesis
Navab et al14,15 presented experimental evidence to support the LDL seeding mechanism. Their work and other experiments also showed that the action of normal HDL particles on LDL particles effectively deactivates the atherogenic load of the LDL particles. It has also been shown that reverse transport processes participate in the elimination of this atherogenic load. Moreover, reverse transport of lipid hydroperoxides, in particular, CEOOH is, as mentioned earlier, much more effective than that of native phospholipids and cholesterol esters.121 It would therefore follow that the PON-1 enzymatic activity for elimination of lipid hydroperoxides and CEOOH and their elimination by reverse cholesterol transport would contribute synergisti decrease in cardiovascular risk.
We attempted to address this question through analysis of polymorphic alleles described for the CLA1 gene (exons 1 and 8 and intron 5) by assigning them a measurable phenotype and determining an associated cardiovascular risk. Then, in a second phase, we assessed whether any association had occurred with some of the allelic variants described for the PON1 gene. We were able to confirm the presence of this genetic synergy. We also found that some of the allelic variants of CLA-1 modulated the plasma content of CEOOH. However, we did not obtain similar phenotypic evidence associated with PON1 polymorphisms.123
CONCLUSIONS
An atheroprotective role linked to reverse transport should be considered in addition to the antioxidative effect of different enzymes associated with HDL particles. The identification of multiple substrates for the paraoxonase-1 enzyme has extended the perspective of vascular protection mediated by HDL particles, although the exact molecular mechanisms by which this occurs have yet to be identified. The characterization of the CLA-1 receptor, its function, and its possible role as a determinant of the variation of plasma concentrations of oxidized lipids contributes to the knowledge of HDL metabolism and suggests the need for more comprehensive approaches in human studies, as well as the possibility of new therapeutic targets.
ACKNOWLEDGMENTS
The authors thank the work done by the technician Ms Lidia Estupiñán-Quintana.
This study was partially funded with a research grant from the Instituto de Salud Carlos III (FIS 01/0190) and FUNCIS 6/2002 (and extension).
Correspondence: Prof. J.C. Rodríguez Pérez.
Unidad de Investigación-Nefrología. Hospital Universitario de Gran Canaria Doctor Negrín.
Barranco de la Ballena, s/n. 35010 Las Palmas de Gran Canaria. España.
E-mail: jrodperd@gobiernodecanarias.org