The guidelines of the European Society of Cardiology (ESC) are endorsed by the Spanish Society of Cardiology (SEC) and translated into Spanish for publication in Revista Española de Cardiología. Each new guideline is accompanied by a commentary written in accordance with the objectives and methodology recommended by the Clinical Practice Guidelines Committee of the SEC.1 The Guidelines Committee appointed a group of experts to draft the present article discussing the new 2017 ESC/EACTS guideline for the management of valvular heart disease.2 Subsequently, the Clinical Cardiology, Imaging, and Cardiac Catheterization Sections appointed other experts, who have made important contributions to the document presented here.
These guidelines are an update of those published in 2012.3 Important advances have been made in the last 5 years, justifying the publication of new guidelines, with relevant changes from the previous document: a) there is a specific section on atrial fibrillation in valvular heart disease, with special attention given to the role of direct oral anticoagulants; b) new evidence has been obtained on transcatheter aortic valve implantation (TAVI) for the treatment of severe aortic stenosis; c) criteria are established that may be useful in the diagnosis of low-gradient severe aortic stenosis; and d) new antithrombotic therapy indications are proposed for patients with surgical and percutaneous prostheses.
The current guidelines, similar to the previous document, suffer from a lack of evidence to support the recommendations made. There is a dramatic increase in recommendations, from 70 to 159, although many of them are IIb C. In addition, there are 2 new level A recommendations: the indication for surgical ablation in patients with symptomatic atrial fibrillation undergoing valve surgery and the possibility of dual antiplatelet therapy instead of triple therapy in patients with a mechanical prosthesis after acute coronary syndrome and stent implantation (with hemorrhagic risk more important than ischemic risk). Most recommendations are still level C (123 [77%]). We appreciate the inclusion in these guidelines of a section specifying both the changes from the previous guidelines3 and the new recommendations. We consider the guidelines to be an irreplaceable tool for the evaluation of patients with valvular heart disease because they provide all of the relevant information on the disease with a highly didactic approach to the topics, particularly the algorithms for the management of the different valvular heart diseases.
GENERAL COMMENTSThe document aims to be a general guideline for the management of patients with valvular heart disease but stresses that decision-making should be individualized for each patient by taking into account the resources available in each center, both diagnostic and therapeutic, and, of course, the wishes of the patient. It insists on the need for decisions to be made within a multidisciplinary or heart team, especially in the case of high risk or asymptomatic patients.
Clinical Evaluation and Risk StratificationThe attention given to clinical evaluation aspects has drastically decreased in this guideline to underscore the adequate stratification of patients’ surgical risk through various scores, mainly the EuroSCORE II and STS models. EuroSCORE I is set aside because it overestimates surgical risk. Other risk factors not included in these models should be evaluated, such as frailty, porcelain aorta, and previous exposure to chest radiation. In elderly patients, it is essential to evaluate both comorbidity and lung disease, chronic kidney disease (glomerular filtration rate < 30mL/min), and cerebrovascular disease, which undoubtedly increase mortality for both surgical and percutaneous interventions. This increased interest in risk assessment is partly motivated by the exponential growth in percutaneous treatment, especially for aortic stenosis.
The guidelines introduce the concept of a “heart valve center” (or center of excellence in valvular heart disease), which comprises a multidisciplinary team that must meet regularly to discuss complex cases, follow intervention protocols, receive consultations from other centers, avail itself of all noninvasive diagnostic imaging techniques, and include highly experienced cardiac surgery and interventional cardiology offering all surgical or catheter interventional possibilities. The advisability of the centralized performance of highly complex surgical or percutaneous interventions (eg, repairs, MitraClip implantation, TAVI) is emphasized, because a learning curve needs to be overcome and a minimum volume of cases per center are required to maintain quality of care. The results of these “heart valve centers” must be audited and be available for internal and external evaluation.
Imaging TechniquesEchocardiography remains the main technique for the initial diagnostic approach because it permits elucidation of the etiological mechanisms and hemodynamic impact and, in many cases, determination of the prognosis. The guidelines confirm the use of transesophageal echocardiography to evaluate the results of surgical and percutaneous interventions. Regarding the indications for other imaging techniques, the guidelines offer few novelties. The authors stress the usefulness of computed tomography (CT) in the selection of candidates for TAVI and as an alternative to invasive coronary angiography to rule out coronary disease, although only for patients with low atherosclerotic risk. Magnetic resonance imaging (MRI) is considered useful when echocardiographic studies are not optimal and as the reference technique in the assessment of right ventricular size and function.
Management of Associated ConditionsRegarding the management of associated coronary disease, the guidelines newly recommend that patients with coronary disease in proximal segments undergo percutaneous coronary intervention before TAVI or MitraClip implantation (IIa). For patients with atrial fibrillation, the document introduces the possibility of direct oral anticoagulant use for more than 3 months after the intervention in patients with a bioprosthesis and in those with native valve disease, except if there is moderate or severe mitral stenosis (indication IIa, evidence C).
Questionable aspects- •
More objective tools should be developed to stratify the risk of patients for valve surgery vs catheter intervention. Futility should also be avoided.
- •
Because the new CT equipment, increasingly available and with a greater number of detectors, obtains images of excellent quality with minimal radiation doses and with a good correlation with invasive coronary angiography, its use could be justified in patients with an increased risk of atherosclerotic disease (at least moderate).
- •
MRI is the reference technique for assessing the size and function of both ventricles and can provide variables that allow for a more adequate prediction of these patients’ outcomes.
- •
The indication for direct oral anticoagulants in the first 3 months after biological prosthesis implantation or TAVI is not clearly established. Likewise, its contraindication in patients with moderate or severe mitral stenosis is not linked to any study.
The guidelines reaffirm the role of echocardiography in the study of morphological and valvular alterations and the regurgitation mechanism and the quantification of regurgitation severity and ventricular function. The echocardiographic information is useful to determine the possibility of valve repair. Importantly, it is clearly explained how the aortic root and ascending aorta should be measured (from the leading edge to the leading edge of the aortic wall at end-diastole).4 Depending on the results of these measurements, the ascending aorta is classified into 3 phenotypes: aortic root aneurysm, ascending aortic (or tubular) aneurysm, or without aortic aneurysm (isolated aortic regurgitation). Although MRI is an adequate technique for measuring the ascending aorta, the guideline recommends multidetector CT (MDCT) with ECG synchronization when surgery is proposed, due to its better temporal resolution. With CT and MRI, the diameters must be measured from the inner edge to the inner edge at end-diastole and using the double-oblique technique, perpendicular to the wall of the vessel, in each segment. The aortic sinus should be measured from sinus to sinus and not from the sinus to the opposite commissure.
The indication for valve surgery in aortic regurgitation (AR) is the presence of symptoms or a documented ejection fraction (EF) ≤ 50% (both, indication I B). It should also be considered (IIa B) for asymptomatic patients with an EF > 50% and a severely dilated left ventricle with end-diastolic diameter > 70mm or end-systolic diameter > 50mm (> 25mm/m2), especially if the body surface area is less than 1.68 m2. As novelties, the possibility of valve repair in selected patients is included, mainly those with root dilatation (type I mechanism) or cusp prolapse (type II) and always after discussion of the case in the multidisciplinary team (class I, evidence C). The indication for this type of surgery would lead to referrals to centers specialized in this type of surgical repair. An aortic intervention is still recommended in patients requiring valve surgery who have an aortic root or ascending aorta ≥ 45mm. In Marfan syndrome, surgery is indicated for diameters ≥ 50mm, or ≥ 45mm when there are additional risk factors, including hypertension or growth > 3mm per year (in the previous guidelines, the threshold was 2mm, which bordered on the variability of the technique itself). The correct comparison of the measurements in the studies is important, using the same methodology, projection, and level for the aortic measurement. For patients with a TGFBR1 or TGFBR2 mutation (including Loeys-Dietz syndrome), surgery is indicated with aortic diameters ≥ 45mm. Regarding the medical treatment for aortic dilation, the guideline adds losartan for patients with Marfan syndrome as an alternative to beta-blockers.
Questionable Aspects- •
The criteria for aortic valve repair or replacement are poorly defined and should be better established in the coming years.
- •
MRI is a technique with greater utility than reflected in the guidelines. It is sometimes difficult to define AR severity by Doppler echocardiography, especially in the presence of eccentric regurgitant jets. The regurgitant fraction measured by MRI is a predictor of symptom development and need for surgery.
- •
Comprehension of the treatment algorithm for AR is hampered by its initiation with significant dilatation of the aorta, ignoring regurgitation severity. In the absence of significant AR, the indications for surgery of ascending aortic dilatation should be developed in the guidelines for aortic disease to avoid overlapping criteria or their differences.
- •
The guidelines create some confusion by considering that, in the case of women with a small body surface area, mutations in TGFBR2, or extra-aortic alterations (phenotypic aspects), an intervention can be considered with aortic diameter values ≥ 40mm. In fact, the study used as the basis for this indication for early surgery required the presence of significant extra-aortic alterations.7
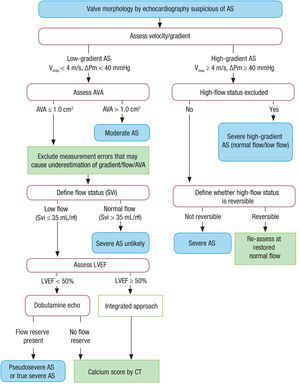
Although echocardiography is the gold standard for the diagnosis and quantification of aortic stenosis (AS), and the aortic valve area (AVA) is the ideal parameter to quantify its severity, there are technical limitations for its use in clinical decision-making. The AVA should be considered together with stroke volume, mean gradient (the most robust parameter), ventricular function, hypertrophy, degree of calcification, and blood pressure. Four categories of AS with an AVA < 1 cm2 are defined: a) AS with mean gradient > 40mmHg, which is considered normal; b) low-flow AS (stroke volume index ≤ 35mL/m2) and gradient < 40mmHg with EF ≤ 50%, with severity defined by stress echocardiography with dobutamine; c) AS with mean gradient < 40mmHg, with EF > 50% but stroke volume index ≤ 35mL/m2), with measurement errors systematically ruled out and severity confirmed by CT or other techniques; and d) low-gradient, normal flow, and AS with normal EF, which are considered moderate AS (Figure).
Stepwise integrated approach for the assessment of aortic stenosis severity, considering valve area, stroke volume, and ejection fraction. A high-flow status can be reversible in situations such as anemia, hyperthyroidism, and arteriovenous shunts. Pseudosevere aortic stenosis is considered to be present when valve flow normalization increases the aortic valve area to > 1.0 cm2. ΔPM, mean transvalvular pressure gradient; AS, aortic stenosis; AVA, aortic valve area; CT, computed tomography; LVEF, left ventricular ejection fraction; SVi, stroke volume index; Vmax, maximum velocity.
In the diagnosis of low-flow, low-gradient AS with preserved EF, of particular importance is an integrated assessment of various clinical variables with different diagnostic methods.7 Thus, AS is unlikely to be serious when the calcium score is > 1600 AU in men and > 800 AU in women; in addition, in these patients, an AVA cutoff of ≤ 0.8 cm2 is proposed. Eighteen therapeutic indications are made, with an increased number of class I recommendations (55%) and a single class III recommendation; 78% of the recommendations are supported by a randomized study. In severe symptomatic low-gradient and low-flow AS without contractile reserve, the grade of recommendation has been changed in favor of surgery from IIb to IIa for patients with a high calcium score (> 3000 AU in men and > 1600 AU in women).
The role of clinical follow-up at short 6-monthly intervals is reinforced for severe asymptomatic AS. Valve replacement indications based on very severe stenosis or accelerated hemodynamic progression are maintained, such as a Vmax > 5.5 m/s, severe valve calcification, and annual Vmax progression of ≥ 0.3 m/s/yr. A new IIa C indication is defined for the presence of severe pulmonary hypertension (pulmonary artery systolic pressure > 60mmHg at rest confirmed using an invasive method) without other explanations. The IIb C indication is also modified for repeat and markedly elevated concentrations of natriuretic peptides (> 3 times the normal range for the patients’ age and sex) without any other possible explanation, becoming a IIa C recommendation. In addition, the guidelines eliminate 2 previous IIb indications, namely, an increase in the mean pressure gradient > 20mmHg with exercise and excessive left ventricular hypertrophy in the absence of hypertension.
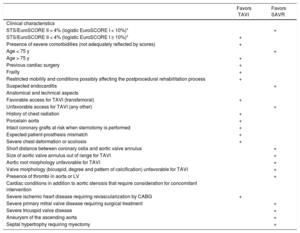
The choice of therapeutic modality (percutaneous approach or sternotomy) provides the greatest number of novelties, and 5 class I recommendations are established: a) cardiac surgery and cardiology should be available in the same center; b) the decision should be made by individually assessing the technical aspects and benefit/risk balance of each modality, taking into account the experience and local results; c) valve surgery is preferred for low-risk patients or those without additional factors not included in the scales, such as frailty, porcelain aorta, and previous radiation; d) for patients with intermediate or high surgical risk or with additional factors, the percutaneous approach should be weighed up by considering patients’ age and the possibility of femoral access; and e) TAVI is indicated for patients considered unsuitable for surgery by the multidisciplinary team. The table specifies the aspects to consider in the indication for TAVI. Balloon valvuloplasty is considered only a bridge to valve replacement or a diagnostic option. A new IIa C indication for valve-in-valve TAVI is included for patients with prosthetic dysfunction, depending on the patients’ surgical risk and the type and size of the prosthesis. Likewise, a new IIa C recommendation is established for the possibility of percutaneous coronary revascularization in candidates for TAVI who have proximal coronary stenosis > 70%. Importantly, the guideline incorporates TAVI data from patients with intermediate risk, based on the results of the PARTNER-II and SURTAVI studies and different meta-analyses.8,9 This evidence shows that, in elderly patients with intermediate surgical risk, TAVI is non-inferior to surgery in terms of mortality and is even superior with transfemoral access.
Aspects to Be Considered by the Multidisciplinary Team When Deciding Between Surgical Aortic Valve Replacement and TAVI for Patients with High Surgical Risk
| Favors TAVI | Favors SAVR | |
|---|---|---|
| Clinical characteristics | ||
| STS/EuroSCORE II < 4% (logistic EuroSCORE I < 10%)* | + | |
| STS/EuroSCORE II < 4% (logistic EuroSCORE I ≥ 10%)* | + | |
| Presence of severe comorbidities (not adequately reflected by scores) | + | |
| Age < 75 y | + | |
| Age > 75 y | + | |
| Previous cardiac surgery | + | |
| Frailty | + | |
| Restricted mobility and conditions possibly affecting the postprocedural rehabilitation process | + | |
| Suspected endocarditis | + | |
| Anatomical and technical aspects | ||
| Favorable access for TAVI (transfemoral) | + | |
| Unfavorable access for TAVI (any other) | + | |
| History of chest radiation | + | |
| Porcelain aorta | + | |
| Intact coronary grafts at risk when sternotomy is performed | + | |
| Expected patient-prosthesis mismatch | + | |
| Severe chest deformation or scoliosis | + | |
| Short distance between coronary ostia and aortic valve annulus | + | |
| Size of aortic valve annulus out of range for TAVI | + | |
| Aortic root morphology unfavorable for TAVI | + | |
| Valve morphology (bicuspid, degree and pattern of calcification) unfavorable for TAVI | + | |
| Presence of thrombi in aorta or LV | + | |
| Cardiac conditions in addition to aortic stenosis that require consideration for concomitant intervention | ||
| Severe ischemic heart disease requiring revascularization by CABG | + | |
| Severe primary mitral valve disease requiring surgical treatment | + | |
| Severe tricuspid valve disease | + | |
| Aneurysm of the ascending aorta | + | |
| Septal hypertrophy requiring myectomy | + |
EuroSCORE, European System for Cardiac Operative Risk Evaluation; LV, left ventricle; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
* STS, EuroSCORE II, and logistic EuroSCORE I10 scores (http://www.euroscore.org/calcge.html); (the models have major limitations for their practical use in this setting by insufficiently considering valve disease severity and omitting major risk factors such as frailty, porcelain aorta, and chest radiation). EuroSCORE I markedly overestimates 30-day mortality and should be replaced by EuroSCORE II; however, it has been provided here for comparison because it has been used in many TAVI studies/registries and may still be useful to identify the patient subgroups for a decision between intervention modalities and to predict 1-year mortality.
- •
The limitations of AVA calculation by echocardiography using the continuity equation are increasingly evident. The guidelines propose the inclusion of several clinical and imaging variables to diagnose severe AS. However, this vitally important aspect does not seem set in stone.
- •
It remains unclear how to identify asymptomatic patients who would benefit from an earlier surgical treatment.
- •
There is still a need to establish new indications for TAVI in patients younger than 75 years or with lower surgical risk. In addition, the durability of TAVI prostheses is still poorly established.
The new guidelines propose substantial changes in the management of mitral regurgitation (MR) and maintain similar recommendations for mitral stenosis, a pathology with few new data in recent years. More frequent echocardiographic follow-up (every 6 months) is recommended in patients with severe MR (previously, it was annual) and ideally in the context of a “heart valve center”, or echocardiogram every 1 to 2 years for patients with moderate MR (previously, every 2 years).
The guidelines maintain the surgical indication for patients with severe symptomatic or asymptomatic primary MR with EF ≤ 60%, end-systolic diameter ≥ 45mm, atrial fibrillation, or systolic pulmonary pressure ≥ 50mmHg confirmed by hemodynamic monitoring. However, changes have been introduced in 2 aspects: a) a tendency to operate on asymptomatic patients with severe primary MR, and b) indications are established for treatment with percutaneous devices. Thus, surgery (IIa indication) is recommended in asymptomatic patients with severe MR even when they are in sinus rhythm and have a left ventricular (LV) EF > 60% if the probability of repair is high, the LV end-systolic diameter is between 40 and 44mm, and one of the following criteria is met: the left atrium is dilated (≥ 60mL/m2) or the cause is chordae tendineae rupture. The indication is removed for surgery in the case of severe pulmonary hypertension with exercise in patients with asymptomatic primary MR.
In the quantification of secondary MR, usually a consequence of ischemic heart disease, cardiomyopathy, or chronic atrial fibrillation, the guidelines mention that, in the severity criterion, although the orifice or regurgitant volume values are lower than in primary MR, the prognosis of these patients may be more closely related to ventricular dysfunction than to MR severity. Percutaneous devices are incorporated into the treatment of severe secondary MR (IIb recommendation). For patients with severe ventricular dysfunction who are not indicated for revascularization and remain symptomatic despite optimal medical therapy, surgery may be indicated if the surgical risk is low, and percutaneous procedures if the surgical risk is not low and valve morphology is favorable (IIb C), in particular with LVEF > 30%. The choice between surgery and percutaneous repair will depend on the surgical risk. For patients with LVEF < 30%, the indication must be individualized because there is no evidence that a reduction in secondary MR improves survival. The indication of the previous guideline3 for treatment of moderate MR during coronary revascularization surgery has been withdrawn, although it is maintained if the MR is severe.
There are no substantial changes to mitral stenosis management. Valve replacement surgery should be considered for asymptomatic patients, those with unfavorable characteristics for percutaneous mitral commissurotomy in the case of high embolism risk or hemodynamic decompensation, or if symptoms develop with low levels of exertion, as long as the surgical risk is low.
Questionable Aspects- •
Although a recommendation for surgery is made in patients with asymptomatic MR and a significantly dilated atrium, there are still substantial differences from the American recommendations,11 in which repair is only performed if it is feasible and has high rates of success and durability. No light is shed on the potential usefulness of repeat measurements showing progressive dilatation or an LVEF drop that does not reach the cutoff values described in the recommendations.
- •
In secondary severe MR, the present guidelines incline toward valve repair with restrictive annuloplasty as the technique of choice. However, based on a recent randomized study,12 the latest update of the American guidelines recommends valve replacement surgery with preservation of the subvalvular apparatus due to a higher rate of MR recurrence after repair.
- •
With regard to percutaneous repair (MitraClip) of secondary MR, despite multiple registries and meta-analyses, the IIb recommendation with level of evidence C remains unchanged.
This guidelines do not include relevant changes regarding tricuspid valve disease compared with that of 2012.3 They do include the possibility of tricuspid valve repair for patients undergoing left valve surgery with moderate tricuspid regurgitation, if right heart failure has been documented, without the need for the presence of dilatation of the valve annulus or right ventricular dysfunction (IIb C).13
COMBINED AND MULTIPLE VALVE DISEASESIn the case of combined valve lesions, the gradient is considered to better reflect the hemodynamic load imposed by the valve lesion, in preference to the valve area and other measurements. Valve repair is considered the procedure of choice and the new guidelines remove a paragraph that favored the implantation of 2 prostheses if one of the valves was not repairable.
PROSTHETIC VALVESOne of the most important novelties vs the previous guidelines3 is in the section related to the antithrombotic management of patients with prostheses. Accordingly, the number of recommendations increases from 8 to 18. These include recommendation I with level of evidence B for INR self-management and recommendation IIa for dual antiplatelet therapy in the first 3 to 6 months after TAVI and then a single antiplatelet agent.
Emphasis is placed on the need to adequately inform patients about the risks and benefits of a mechanical or biological prosthesis implantation and the importance of a joint decision with the patient, with age not the only consideration. The document makes it very clear that direct oral anticoagulants should not be given to patients with mechanical prostheses and that the objective should still be the median INR, and not a range, to avoid the possibility that extreme values within the therapeutic range be considered valid. An important aspect is the new recommendations for the antiplatelet therapy of patients with mechanical valve prostheses after the implantation of a coronary stent14 with the 1-month use of triple antiplatelet therapy with vitamin K antagonists (VKAs), aspirin, and clopidogrel, regardless of the type of stent and clinical syndrome motivating the implantation, and to prolong the therapy for up to 6 months in patients with high ischemic risk but without high bleeding risk. Dual therapy with clopidogrel and VKAs should be considered as an alternative to triple therapy in patients with higher bleeding risk; discontinuation of antiplatelet therapy can be considered 12 months after stent implantation. For patients with mechanical prostheses with concomitant atherosclerotic disease, the addition of low-dose aspirin to VKAs has been changed from a IIa to IIb indication.
The IIa indication is maintained for treatment with VKAs in the first 3 months after implantation of a bioprosthesis in the mitral or tricuspid position, or aspirin alone for aortic interventions. Despite the absence of confirmatory studies, the interesting role of direct oral anticoagulants in biological prostheses is considered, especially after the third month when anticoagulation is indicated. The recommendation to implant a mechanical prosthesis in already anticoagulated patients and carriers of another mechanical prosthesis has been relegated from a class I to class IIa indication.
There have been no changes in the management of obstructive thrombosis of mechanical prostheses. Emergency surgical replacement is the treatment of choice when there are no significant comorbidities (class I indication). Fibrinolysis should be considered when surgery is unavailable or there is high surgical risk (IIa indication). For nonobstructive prosthetic thrombosis after an embolic event, surgery is indicated (IIa) when the thrombus is ≥ 10mm.
Reoperation is recommended in patients with paravalvular leak due to endocarditis that causes severe hemolysis or symptoms, with the possibility of percutaneous closure for patients with high surgical risk (decided by the multidisciplinary team), and an individualized indication is proposed for percutaneous implantation of a new aortic bioprosthesis (valve-in-valve), depending on risk and prosthesis type and size.
Questionable Aspects- •
In clinical practice, patients receiving a biological prosthesis tend to be younger than those receiving mechanical ones. This trend is probably due to the more active lifestyle of the middle-aged population, who prefer to avoid anticoagulation with VKAs, and to the greater durability of some biological prostheses. The guidelines miss the opportunity to discuss the types of mechanical and biological prostheses available on the market and their differences in terms of hemodynamics, thrombosis risk, and durability.
- •
Although recent randomized studies have proposed lowering the INR target in patients with mechanical aortic prostheses, the recommendations have not been changed. These data should be confirmed in the future.
- •
After the percutaneous or surgical implantation of a biological prosthesis, the current guidelines recommend echocardiography at 30 days, 1 year, and annually thereafter (previously, for patients with normal biological prostheses, only an annual echocardiogram was recommended after the fifth year). These recommendations are not based on any clinical study and would lead to a significant overburden of echocardiography laboratories in Spain.
- •
The antithrombotic therapy for the first 36 months after TAVI is poorly defined. The possible use of a single antiplatelet agent instead of dual antiplatelet therapy is proposed, as well as the possible benefit of initial anticoagulation in patients with low bleeding risk to avoid subclinical thrombosis.15
- •
Management of nonobstructive thrombosis of a biological prosthesis with an embolic event and a thrombus < 10mm is poorly defined. Equally, the strategy is not specified if the thrombus persists despite adequate anticoagulation.
Pregnancy should be discouraged in 4 situations: severe mitral stenosis, severe symptomatic AS, Marfan syndrome with aorta > 45mm, and Turner syndrome (diameter > 27.5mm/m2). Recommendations for cesarean section are also made. No indication is provided for surgery of a bicuspid valve with aortic dilatation.
As for anticoagulation in patients with a mechanical prosthesis, oral anticoagulation is recommended until delivery in women requiring warfarin < 5mg/d and a switch to low-molecular-weight heparin while monitoring the anti-Xa activity in the remaining patients.
Questionable Aspects- •
In relation to women with a mechanical prosthesis receiving oral anticoagulation during pregnancy, it should be specified that oral anticoagulation is to be maintained until the 36th week of pregnancy, at which time it should be changed to low-molecular-weight heparin.
As a consequence of these guidelines, the creation of multidisciplinary units for valvular heart disease should be considered in tertiary centers, as well as the promotion of referral centers for autonomous communities. Adherence to the recommendations may require some improvements in quality standards in clinical treatment, imaging techniques, and surgical or percutaneous treatment. The recommendation for annual revisions in certain subgroups of valvular heart disease patients constitutes an important care overburden, difficult to handle in the current environment. With tools such as telemedicine, close and fluid clinical collaboration with primary care physicians should be encouraged, especially for patients whose mid-term risk of complications is not foreseeable.
LOCAL SOCIOECONOMIC IMPLICATIONSAdvances in the treatment of valvular heart disease, particularly in the field of surgical and percutaneous treatment, represent a significant increase in health expenditure, occasionally in a group of seriously ill patients. Spanish professionals cannot ignore our responsibility to analyze and individualize the benefit/cost balance of our actions. Specialized multidisciplinary referral centers could undoubtedly permit an objective analysis of complex valve disease patients and improve decision-making efficiency. These units, in addition to optimizing the care of these patients in the hospital environment, need to communicate with regional hospitals and family physicians in their catchment area.
CONCLUSIONSThese guidelines are an update of the recommendations for the management of patients with valvular heart disease and are of considerable use in the practice of the general clinical cardiologist. Promotion and description of the requirements of multidisciplinary units for valve disease patient care are increasingly required, given the continuous increase in therapeutic options for complex, older, and more comorbid patients. In this regard, the guidelines include the contributions of recent studies of TAVI, consider the indication for earlier surgery for primary MR, which can be reliably repaired, and address in greater depth the antithrombotic management of patients with valvular prostheses.
CONFLICTS OF INTERESTNone declared.
APPENDIX. AUTHORSSEC Working Group for the 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Arturo Evangelista, J. Alberto San Román, Francisco Calvo, Ariana González, Juan José Gómez Doblas, Ana Revilla, Juan Antonio Castillo, and Carlos González Juanatey.
Expert Reviewers for the 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Juan José Gómez Doblas, Teresa López Fernández, Manuel Barreiro, María José Oliva, Laura Galian Gay, Ana Serrador, Pilar Jiménez Quevedo, Manuel Pan, Miguel A. Arnau Vives, Javier López Díaz, and Xabier Borrás Pérez.
SEC Guidelines Committee: Alberto San Román, Fernando Alfonso, Arturo Evangelista, Ignacio Ferreira-González, Manuel Jiménez Navarro, Francisco Marín, Leopoldo Pérez de Isla, Luis Rodríguez Padial, Pedro Luis Sánchez Fernández, Alessandro Sionis, and Rafael Vázquez García.
.
SEC Working Group for the 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease, Expert Reviewers for the 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Diseases, and the SEC Guidelines Committee◊,*.
The names of all authors of this article are listed in the Appendix.