To the Editor:
We present a case that illustrates the usefulness of cardiac magnetic resonance (CMR) in amyloid heart disease.
A 71-year-old woman with diabetes went to the emergency room for syncope of 2 months' evolution, with 2-3 daily episodes but no focal neurological deficits. The examination revealed pallor of the skin and mucosa and a grade II/VI apical systolic murmur. Blood pressure and heart rate in decubitus and standing position were 130/70 mm Hg and 68 bpm, and 80/40 mm Hg and 74 bpm, respectively. The rest of the examination was normal.
The analyses indicated normochromic, normocytic anemia; elevated urea, creatinine, and sedimentation rate; hypoalbuminemia, hypergammaglobulinemia with monoclonal peak; elevated immunoglobulin G; and enlarged lambda chains in serum and urine.
The electrocardiogram showed sinus rhythm, horizontal axis, and low voltage. Mild cardiomegaly was observed on the chest x-ray. The echocardiogram, which was suboptimal due to an inadequate acoustic window, presented concentric left ventricular hypertrophy with no granular sparkling, normal systolic function, and diastolic dysfunction due to slow relaxation.
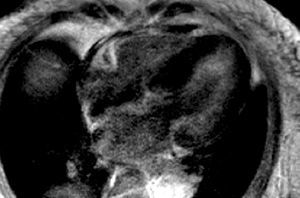
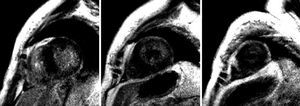
Since amyloidosis was suspected based on the clinical symptoms and the echocardiography was inconclusive, CMR was performed with anatomical and functional sequences in standard views, a TSE-T1 sequence in the four-chamber view and, following administration of a gadolinium-DTPA bolus (0.1 mmol/kg), inversion-recovery FLASH sequences and TI-scout sequences.1 Amyloidosis was strongly suggested by the study findings, which included: a) diffuse myocardial thickening, decreased volumes, and loss of longitudinal systolic function; b) slight thickening of the right ventricular free wall and interatrial septum; c) aortic valve thickening, mild mitral and aortic regurgitation; d) diffuse, subendocardial late enhancement, mainly in the lateral LV wall (Figures 1 and 2), accelerated gadolinium kinetics in blood and myocardium, with subendocardial T1 of 510 ms 4 min following gadolinium administration; and e) mild pericardial and pleural effusion.
Figure 1. Inversion-recovery sequence after administration of a gadolinium-DTPA bolus, 4-chamber view. Diffuse myocardial thickening and diffuse subendocardial gadolinium enhancement are observed, mainly in the lateral wall of the left ventricle.
Figure 2. Identical sequence as in the previous figure, basal, mid, and apical short-axis views. More intense diffuse subendocardial gadolinium enhancement in the side wall of the left ventricle is evident.
Based on these findings, a sural nerve biopsy was performed and primary amyloidosis was diagnosed. Because of the patient's age and the fact that she was not eligible for bone marrow transplantation, melphalan and prednisone therapy was initiated. The patient died some months later of progressive heart failure.
Amyloidosis is a complex systemic disease characterized by extracellular deposits of amyloid fibrillary protein in the body.2 The diagnosis of this condition is usually established in advanced stages; structural heart disease is the most frequent cause of death.3 Treatment for primary amyloidosis is unsatisfactory, although some improvement in survival and functional status has been attained with alkylating agents.4 The patient did not respond well in this case, probably due to the advanced state of the disease.
Doppler echocardiography, the initial imaging technique, can detect wall thickening with small chambers, dilated atria, valvular and interatrial septum thickening, and diastolic and systolic dysfunction.5-7 On occasions, myocardial thickening is not uniform and the features resemble hypertrophic cardiomyopathy, although in amyloidosis the electrocardiogram usually exhibits low voltage. The granular sparkling appearance caused by the deposits may be difficult to visualize. In the present case, the echocardiogram actually did not detect the granular texture, or the severe valve regurgitation or pericardial effusion, possibly because they were mild or had not yet appeared at the time.
Cardiac magnetic resonance can provide more complete information for the diagnosis of this entity. In addition to the morphological findings described on echocardiography, amyloid deposits specifically affect the gadolinium kinetics in the blood and myocardium.1 Following gadolinium administration, there is greater shortening of the subendocardial T1 and a larger difference between subendocardial T1 and blood; hence a cut-off point can be established with good diagnostic accuracy. In addition, there is a typical pattern of diffuse late subendocardial enhancement, which reflects the greater infiltration of amyloidosis in the subendocardium with expansion of the extracellular space.1