No specialty in the history of medicine has seen such rapid growth and innovation as interventional cardiology, due to a combination of the compelling need for better results in the treatment of coronary artery disease (CAD), the first cause of death worldwide, and the unique personality of remarkable individuals driving progress.
Since its first application in peripheral atherosclerosis in 1963 and the first coronary angioplasty in 1977, the field of interventional cardiology has evolved and expanded enormously, with percutaneous transluminal coronary angioplasty being one of the most common procedures performed in contemporary clinical practice. In this editorial, we provide an overview of the development of coronary stents since their introduction in the late 1980s, with special focus on currently available stents and bioresorbable scaffolds.
WHY IS PLAIN BALLOON ANGIOPLASTY NOT ENOUGH?In 1963, Dotter and his trainee, Judkins, accidentally “recanalized” an occluded iliac artery while performing an abdominal aortogram. One year later, they intentionally used a catheter for the first successful percutaneous transluminal peripheral angioplasty. More than a decade later, in 1977, Gruentzig performed the first balloon percutaneous transluminal coronary angioplasty (or POBA, plain old balloon angioplasty, as it was later called) in a conscious man, starting a revolution in the treatment of CAD.
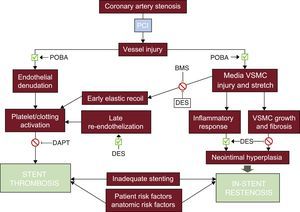
Plain old balloon angioplasty can transiently achieve a larger luminal diameter by plaque extrusion, but elastic recoil rapidly obliterates this gain. Plastic, more durable, changes can be achieved with plaque dissection, but this mechanism carries potential risks of acute vessel closure. Abrupt occlusion obliged the pioneers of coronary angioplasty to have an active surgical stand-by during these procedures. The balloon induced intimal denudation and medial tearing exposed subendothelial matrix to blood, promoting platelet aggregation and thrombosis in the acute phase and chronic negative vascular remodeling (late recoil) and neointimal hyperplasia. Insufficient initial gain and restenosis led to a nearly complete loss of the clinical benefit in 30% to 40% of patients in the first 6 to 9 months (Figure 1). These limitations required further technological advancement, resulting in the introduction of coronary artery stents.
Pathophysiology of in-stent restenosis and stent thrombosis. Dilatation of the diseased vessel by POBA causes a mechanical injury of the vessel wall, with subsequent endothelial denudation, mechanical damage to the vascular wall with inflammatory response and fibrosis/neointimal hyperplasia, which are the main mechanisms responsible for in-stent restenosis and acute/late stent thrombosis. BMS and DES may prevent some of these negative processes, but also serve as a stimulus to inflammation and fibrosis. In-stent restenosis and stent thrombosis may also be determined by patient risk factors (ie, diabetes, smoking), anatomical features of the treated vessel (such as heavy calcified lesions, diameter of the vessel, the presence of side branches) or inadequate stenting (strut thickness, stent malapposition, inadequate stent diameter). BMS, bare metal stent; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; VSMC, vascular smooth muscle cells.
Coronary stents were developed to prevent arterial recoil and restenosis after balloon dilatation. Stents can be classified into 3 large families: bare metal stents (BMS), drug-eluting stents (DES), and bioresorbable vascular scaffolds (BRS).
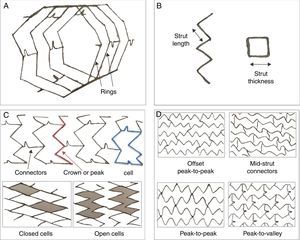
An ideal metallic stent should have good flexibility and deliverability, low thrombogenicity, strong radial force, good radio-opacity under fluoroscopy, and good biocompatibility to ensure low rates of neointimal hyperplasia and stent thrombosis during long-term follow-up (Figure 2). Platinum-chromium, cobalt-chromium or other alloys have largely substituted stainless steel to provide sufficient strength and visibility with thinner struts.
Stent structure and design. A, B, C: Struts, rings, cells, crowns and connectors form the backbone of a stent. Strut: single element that forms larger structural entities (cells, rings and crowns). Cell: small but regularly repetitive structure of a stent, delimited by 2 layers of rings and the connectors and might be open or closed. Connectors: attach the adjacent rings and can be straight or curved or can be direct welds that link the rings directly. Rings and crowns: (1 crown = 2 struts) comprise a cluster of cells and are held together by connectors. D: Orientation of the stent (in-phase or out-of-phase) and connectors (offset peak-to-peak; mid-shaft; peak-to-peak–out-of-phase; peak-to-valley–in-phase). Design and geometry of these components define the mechanical performance of a stent: crowns and rings determine radial support and expansion capacity; the number of connectors is responsible for the longitudinal stability, flexibility, deliverability, side branch access and longitudinal integrity. Open cell designs with a reduced number of connectors provide greater stent flexibility with reduced arterial injury and decreased neointimal response.
A drug-eluting stent has a more complex structure, in general wrapping a polymer coating containing an antiproliferative drug around the stent struts. The polymer may be durable or bioresorbable and some recent stents elute the drug directly. A BRS consists of a platform made of bioresorbable material, either magnesium or poly-L-lactic acid (PLLA), coated with a polymer and an antiproliferative drug.
BARE METAL STENTSIn 1986, Puel and Sigwart set another milestone in the history of PCI by independently implanting the first self-expanding coronary stent (Wallstent, Schneider AG, Bulach, Switzerland). The following year, Palmaz and Schatz developed a balloon-expandable stent (Palmaz-Schatz, Johnson&Johnson, New Brunswick, New Jersey), which became the first Food and Drug Administration (FDA)-approved stent in the United States.
The first stents were made from stainless steel and, despite their thick struts and poor flexibility, showed superiority over POBA, with elimination of abrupt occlusion and a reduced rate of restenosis, confirmed in 2 historic trials published in 1993 (the BENESTENT and the STRESS trials1,2). There was still an obstacle against their universal adoption, the high incidence of acute and subacute stent thrombosis, obliging implanters to use high doses of anticoagulant drugs, leading to unacceptable rates of bleeding. This problem was overcome by the observation with intravascular ultrasound that stents required high pressure for complete expansion and the introduction of dual antiplatelet therapy (DAPT) combining ticlopidine or clopidogrel with aspirin. These stents were still at a significant risk of in-stent restenosis (ISR), reported in 15% to 30% of treated lesions at mid- and long-term follow-up.3
DRUG-ELUTING STENTSSince the identification of neointimal hyperplasia as the major determinant of ISR, the application of antiproliferative agents was the logical answer. Subsequently, in addition to acting as permanent vascular scaffolds, stents soon evolved to become efficient local drug delivery platforms. In 1999, Sousa implanted the first DES in Brazil, signaling the third revolutionary paradigm shift in the history of interventional cardiology.
First-generation Drug-eluting StentsSirolimus and paclitaxel were the 2 antiproliferative drugs initially used in first-generation DESs (respectively CYPHER [Cordis, Milpitas, CA] and TAXUS [Boston Scientific, Marlborough, Massachusetts]). Both were made of stainless steel, had a strut thickness greater than 130μm and have been tested in numerous randomized controlled trials (RCTs) (Table 1),4 showing a significant reduction in ISR, late lumen loss and rate of target lesion/vessel revascularization compared with BMS.7,9,10 The initial enthusiasm was shaken in 2006 when Camenzind published a meta-analysis, showing an increased risk of death and myocardial infarction (MI) related to late and very late stent thrombosis (ST),11 possibly related to delayed endothelialization secondary to antiproliferative drug elution and a hypersensitivity reaction to the polymer coating. Very late ST, although now recognized as a possible complication of first-generation DES, is a rare entity and numerous meta-analyses and data registries have provided reassurance about the use of such devices.12
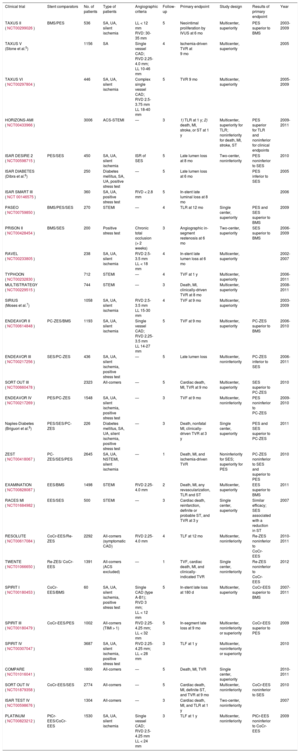
Major Randomized Controlled Trials Comparing Bare Metal Stents With Current-generation Drug-eluting Stents
| Clinical trial | Stent comparators | No. of patients | Type of patients | Angiographic criteria | Follow-up | Primary endpoint | Study design | Results of primary endpoint | Year |
|---|---|---|---|---|---|---|---|---|---|
| TAXUS II (NCT00299026) | BMS/PES | 536 | SA, UA, silent ischemia | LL < 12 mm RVD: 30-35 mm | 5 | Neointimal proliferation by IVUS at 6 mo | Multicenter, superiority | PES superior to BMS | 2003-2009 |
| TAXUS V (Stone et al.5) | 1156 | SA | Single vessel CAD; RVD 2.25-4.0 mm; LL 10-46 mm | 4 | Ischemia-driven TVR at 9 mo | Multicenter, superiority | 2005 | ||
| TAXUS VI (NCT00297804) | 446 | SA, UA, silent ischemia | Complex single vessel CAD; RVD 2.5-3.75 mm LL 18-40 mm | 5 | TVR 9 mo | Multicenter, superiority | 2005-2009 | ||
| HORIZONS-AMI (NCT00433966) | 3006 | ACS-STEMI | — | 3 | 1) TLR at 1 y; 2) death, MI, stroke, or ST at 1 y | Multicenter, superiority for TLR; noninferiority for death, MI, stroke, ST | PES superior for TLR and noninferior for clinical endpoints | 2009-2011 | |
| ISAR DESIRE 2 (NCT00598715) | PES/SES | 450 | SA, UA, silent ischemia | ISR of SES | 5 | Late lumen loss at 8 mo | Two-center, noninferiority | PES noninferior to SES | 2010 |
| ISAR DIABETES (Dibra et al.6) | 250 | Diabetes mellitus, SA, UA, positive stress test | — | 5 | Late lumen loss at 6 mo | PES inferior to SES | 2005 | ||
| ISAR SMART III (NCT 00146575) | 360 | SA, UA, positive stress test | RVD < 2.8 mm | 5 | In-stent late luminal loss at 8 mo | 2006 | |||
| PASEO (NCT00759850) | BMS/PES/SES | 270 | STEMI | — | 4 | TLR at 12 mo | Single center, superiority | PES and SES superior to BMS | 2009 |
| PRISON II (NCT00428454) | BMS/SES | 200 | Positive stress test | Chronic total occlusion (> 2 weeks) | 3 | Angiographic in-segment restenosis at 6 mo | Two-center, superiority | SES superior to BMS | 2006-2009 |
| RAVEL (NCT00233805) | 238 | SA, UA, silent ischemia | RVD 2.5-3.5 mm LL < 18 mm | 4 | In-stent late lumen loss at 6 mo | Multicenter, superiority | 2002-2007 | ||
| TYPHOON (NCT00232830) | 712 | STEMI | — | 4 | TVF at 1 y | Multicenter, superiority | 2006-2011 | ||
| MULTISTRATEGY (NCT00229515) | 744 | STEMI | — | 3 | Death, MI, clinically-driven TVR at 8 mo | Multicenter, superiority | 2008-2011 | ||
| SIRIUS (Moses et al.7) | 1058 | SA, UA, silent ischemia | RVD 2.5-3.5 mm LL 15-30 mm | 4 | TVF at 9 mo | Multicenter, superiority | 2003-2009 | ||
| ENDEAVOR II (NCT00614848) | PC-ZES/BMS | 1193 | SA, UA, silent ischemia | Single vessel CAD; RVD 2.25-3.5 mm LL 14-27 mm | 5 | TVF at 9 mo | Multicenter, superiority | PC-ZES superior to BMS | 2006-2010 |
| ENDEAVOR III (NCT00217256) | SES/PC-ZES | 436 | SA, UA, silent ischemia, positive stress test | — | 5 | Late lumen loss | Multicenter, noninferiority | PC-ZES inferior to SES | 2006-2011 |
| SORT OUT III (NCT00660478) | 2323 | All-comers | — | 5 | Cardiac death, MI, TVR at 9 mo | Multicenter, superiority | SES superior to PC-ZES | 2010 | |
| ENDEAVOR IV (NCT00217269) | PES/PC-ZES | 1548 | SA, UA, silent ischemia, positive stress test | — | 3 | TVF at 9 mo | Multicenter, noninferiority | PES noninferior to PC-ZES | 2009-2010 |
| Naples-Diabetes (Briguori et al.8) | PES/SES/PC-ZES | 226 | Diabetes mellitus, SA, UA, silent ischemia, positive stress test | — | 3 | Death, nonfatal MI, clinically-driven TVR at 3 y | Single center, superiority | PES and SES superior to PC-ZES | 2011 |
| ZEST (NCT00418067) | PC-ZES/SES/PES | 2645 | SA, UA, NSTEMI, silent ischemia | — | 1 | Death, MI, and ischemia-driven TVR | Noninferiority for SES; superiority for PES | PC-ZES noninferior to SES and superior to PES | 2010 |
| EXAMINATION (NCT00828087) | EES/BMS | 1498 | STEMI | RVD 2.25-4.0 mm | 2 | Death, MI, any revascularization, TLR and ST | Multicenter, superiority | EES superior to BMS | 2011 |
| RACES MI (NCT01684982) | EES/SES | 500 | STEMI | — | 3 | Cardiac death, reinfarction, definite or probable ST, and TVR at 3 y | Single center, superiority | Similar efficacy; SES associated with a reduction in ST | 2007 |
| RESOLUTE (NCT00617084) | CoCr-EES/Re-ZES | 2292 | All-comers (symptomatic CAD) | RVD 2.25-4.0 mm | 4 | TLF at 12 mo | Multicenter, noninferiority | Re-ZES noninferior to CoCr-EES | 2010-2011 |
| TWENTE (NCT01066650) | Re-ZES/ CoCr-EES | 1391 | All-comers (ACS excluded) | — | 1 | TVF, cardiac death, MI, and clinically-indicated TVR | Single center, noninferiority | Re-ZES noninferior to CoCr-EES | 2012 |
| SPIRIT I (NCT00180453) | CoCr-EES/BMS | 60 | SA, UA, silent ischemia, positive stress test | Single CAD (type A-B1); RVD 3 mm; LL < 12 mm | 5 | In-stent late loss at 180 d | Multicenter, superiority | CoCr-EES superior to BMS | 2007-2011 |
| SPIRIT III (NCT00180479) | CoCr-EES/PES | 1002 | All-comers (TIMI > 1) | RVD 2.25-4.25 mm; LL < 32 mm | 5 | In-segment late loss at 9 mo | Multicenter, noninferiority or superiority | CoCr-EES superior to PES | 2009 |
| SPIRIT IV (NCT00307047) | 3687 | SA, UA, silent ischemia, positive stress test | RVD 2.25-4.25 mm; LL < 28 mm | 3 | TLF at 1 y | Multicenter, noninferiority or superiority | 2010 | ||
| COMPARE (NCT01016041) | 1800 | All-comers | — | 5 | Death, MI, TVR | Single center, superiority | 2010-2011 | ||
| SORT OUT IV (NCT01879358) | CoCr-EES/SES | 2774 | All-comers | — | 5 | Cardiac death, MI, definite ST, and TVR at 9 mo | Multicenter, noninferiority | CoCr-EES noninferior to SES | 2010 |
| ISAR TEST IV (NCT00598676) | 1304 | All-comers | — | 3 | Cardiac death, MI, and TLR at 1 y | Two-center, noninferiority | 2007 | ||
| PLATINUM (NCT00823212) | PtCr-EES/CoCr-EES | 1530 | SA, UA, silent ischemia | Single vessel CAD; RVD 2.5-4.25 mm LL < 24 mm | 3 | TLF at 1 y | Multicenter, noninferiority | PtCr-EES noninferior to CoCr-EES | 2009 |
ACS, acute coronary syndrome; BMS, bare metal stent; CAD, coronary artery disease; CoCr-EES, cobalt-chromium everolimus -eluting stent; EES, everolimus-eluting stent; ISR, in-stent restenosis; IVUS, intravascular ultrasound; LL, lesion length; MI, myocardial infarction; NSTEMI, non–ST-segment elevation myocardial infarction; PC-ZES, phosphorylcholine-based zotarolimus-eluting stent; PES, paclitaxel-eluting stent; PtCr-EES, platinum-chromium everolimus-eluting stent; Re-ZES, resolute zotarolimus-eluting stent; RVD, reference vessel diameter; SA, stable angina; SES, sirolimus-eluting stent; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TLF, target lesion failure, defined as cardiac death, target-vessel MI, or TLR; TLR, target lesion revascularization; TVF, target-vessel failure, defined as cardiac death, target-vessel MI, or TVR; TVR, target-vessel revascularization; UA, unstable angina; ZES, zotarolimus-eluting stent.
In second-generation DES, the platform was changed to metal alloys (ie, cobalt-chromium or platinum-chromium), which allowed for a reduction in strut thickness and more flexibility (Table 2). Polymers were made of new, more biocompatible molecules such as zotarolimus, everolimus and novolimus (the limus-family drugs), with faster drug elution and subsequent earlier endothelial coverage.
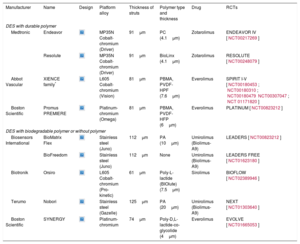
Current Second-generation Drug-eluting Stents With Durable Polymer, With Biodegradable Polymer and Without a Polymer
| Manufacturer | Name | Design | Platform alloy | Thickness of struts | Polymer type and thickness | Drug | RCTs |
|---|---|---|---|---|---|---|---|
| DES with durable polymer | |||||||
| Medtronic | Endeavor | MP35N Cobalt-chromium (Driver) | 91μm | PC (4.1μm) | Zotarolimus | ENDEAVOR IV [NCT00217269] | |
| Resolute | MP35N Cobalt-chromium (Driver) | 91μm | BioLinx (4.1μm) | Zotarolimus | RESOLUTE [NCT00248079] | ||
| Abbot Vascular | XIENCE family* | L605 Cobalt-chromium (Vision) | 81μm | PBMA, PVDF-HPF (7.6μm) | Everolimus | SPIRIT I-V [NCT00180453; NCT00180310; NCT00180479NCT00307047; NCT 01171820] | |
| Boston Scientific | Promus PREMIERE | Platinum-chromium (Omega) | 81μm | PBMA, PVDF-HFP (6μm) | Everolimus | PLATINUM [NCT00823212] | |
| DES with biodegradable polymer or without polymer | |||||||
| Biosensors International | BioMatrix Flex | Stainless steel (Juno) | 112μm | PA (10μm) | Umirolimus (Biolimus-A9) | LEADERS [NCT00823212] | |
| BioFreedom | Stainless steel (Juno) | 112μm | None | Umirolimus (Biolimus-A9) | LEADERS FREE [NCT01623180] | ||
| Biotronik | Orsiro | L605 Cobalt-chromium (Pro-kinetic) | 61μm | Poly-L-lactide (BIOlute) (7.5μm) | Sirolimus | BIOFLOW [NCT02389946] | |
| Terumo | Nobori | Stainless steel (Gazelle) | 125μm | PA (20μm) | Umirolimus (Biolimus-A9) | NEXT [NCT01303640] | |
| Boston Scientific | SYNERGY | Platinum-chromium | 74μm | Poly-D,L-lactide-co-glycolide (4μm) | Everolimus | EVOLVE [NCT01665053] | |
BIOlute, bioabsorbable poly-L-lactide eluting a limus drug; HFP, hexafluoropropylene; L605, cobalt-chromium-tungsten-nickel; MP35N, nickel, cobalt, chromium and molybdenum; PA, polylactic acid; PBMA, poly (n-butylmethacrylate); PC, phosphorylcholine; PVDF, poly-vinylidene fluoride; RCT, randomized controlled trial.
* The XIENCE family includes: XIENCE V, XIENCE nano, XIENCE PRIME, XIENCE PRIME LL, XIENCE Xpedition, XIENCE Xpedition SV, XIENCE Xpedition LL, and XIENCE Alpine.
The safety and efficacy of second-generation DES have been assessed in numerous RCTs, showing significant reductions in rates of MI, target lesion revascularization and ST compared with first-generation DESs.13–15 Given these c inical advances, second-generation DESs have become the most widely used DES worldwide and they are accepted as the percutaneous treatment of choice for CAD, totally replacing BMS and first-generation DES (Table 1).16 However, despite the major technical refinements, concerns persist about their long-term safety. Late and very late ST declined with an incidence of less than 1% at 5 years, which is lower than that of BMS but still represents a concern because of the need to continue DAPT for 1 year and beyond.17,18 The persistence of late events and the attempt to minimize the duration and intensity of the dual antiplatelet regimen have further prompted the development of third-generation devices.
Polymer-free Drug-eluting StentsThe polymer coat is involved in the pathogenesis of long-term stent failure by triggering a potential chronic inflammatory stimulus responsible for delayed endothelial coverage and ST. Therefore, a new strategy to eliminate polymer-mediated complications has been the development of polymer-free DES, which can theoretically avoid these long-term negative effects, decreasing the rate of ST and allowing a shorter duration of DAPT.
However, since the polymer not only acts as a drug carrier, but also modulates the controlled release of the drug over time, the development of polymer-free DES required a new technology to maintain an adequate level of antiproliferative drug over time without the polymer vehicle (Table 2).
Thus, the metallic stent surface was modified to be porous (pores of 5-15nm) and the antiproliferative drug was then directly loaded onto these pores during the DES manufacturing process. However, the drug release was difficult to control and some minor RCTs showed noninferiority but no improvement in clinical outcomes when compared with second-generation DES.19 The drug can also be carried by nanoparticles in a matrix compound, which can facilitate penetration of the drug deeper into vessel walls where they rapidly elute it (Cre8 stent [CID Vascular, Saluggia, VC, Italy] and BioFreedom [Biosensors, Morges, Switzerland]) or applied as micro-drops by crystallization (VESTAsync [MIV therapeutics, Vancouver, Canada]). To date, few RCTs have evaluated the performances of polymer-free DES and larger trials are needed on long-term efficacy and safety. Other third-generation stents appear to achieve the same goal with small biodegradable polymer dots on the abluminal surface of the stent (SYNERGY, Boston Scientific, Minneapolis, Minnesota).
Biodegradable Polymer StentsDrug-eluting stents coated with biodegradable polymers (such as poly-DL-lactide-co-glycolide or PLLA) may offer the benefit of a conventional DES in the early phase and behave as a BMS at later stages.
Degradation of the bioresorbable polymer occurs simultaneously with controlled release of the antiproliferative drug in the early phase after implantation. Following complete elution of the drug and biodegradation of the polymer, only the metallic platform remains in the coronary artery (Table 2). Several bioresorbable polymers are currently used and they differ in biocompatibility, degradation time, and in their different impact on endothelial function, smooth muscle cells growth, and thrombogenicity.20,21
Despite theoretical advantages and encouraging early results, showing lower rates of very late ST than first-generation DESs and noninferiority in terms of efficacy and safety compared with second-generation DESs, long-term results are needed.22,23
Fully Bioresorbable ScaffoldsConcerns over late adverse events related to the persistence of the metallic platforms in the coronary vessel have led to interest in fully bioresorbable stent technology in the past decade, potentially representing the fourth revolution in interventional cardiology. The rationale behind their use is to create a temporary mechanical support in the vessel in order to prevent immediate restenosis and vascular recoil, then allowing it to degrade over time, eliminating the long-term risk associated with the presence of a metallic scaffold.
Referred to as a BRS, these devices provide the local drug delivery and mechanical support of permanent metallic DES in the first 12 months and reabsorb completely after 24 to 36 months, allowing restoration of normal luminal diameter and vasomotor function over the years, removing any nidus for late unfavorable events, potentially reducing the need for long-term DAPT and allowing surgical revascularization if needed.
Bioresorbable scaffolds could be either a metallic alloy (magnesium or iron alloy) or an L-isomer of PLLA polymeric platform, covered with a polymer and an antiproliferative drug. The first drug-eluting BRS was implanted in 1995 and since then approximately 9 BRS have been studied in clinical trials (first-in-man or RCTs), only a few of them have received FDA or CE approval, and the device with the largest and longest experience (BVS ABSORB, ABSORB, Abbott, Minneapolis, MN) has been withdraw from the market.
When an angioplasty is performed with a BRS, the technique used for implantation, the selection of suitable lesions and patients, the pre- and postdilatation technique and the choice of a tailored DAPT are considered crucial to reduce the incidence of ST.24
Despite early optimism, challenges exist for first-generation PLLA BRS. The radial force of a BVS is weaker than the force of DES, so recoil can be a problem because of the rapid absorption. To overcome this problem, stent design requires thick struts to maintain radial strength and they might result in incomplete expansion and reduced lumen diameter after deployment.25 Metallic bioabsorbable stents are becoming attractive since they have the potential to overcome the limitation of biodegradable polymer stents, with more radial force and thinner struts.26
The current-generation of PLLA BRS showed higher rates of device thrombosis and MI at 1 year. These data were confirmed by meta-analyses and clinical registries27 and mainly referred to the Absorb BVS, which is so far the most widely used scaffold and the only one with CE mark and FDA approval. Due to the higher incidence of ST observed with Absorb, Abbott has recently restricted its use to controlled clinical trials or registries. Indeed, there is still a long road ahead before BRS can be routinely used in clinical practice.
DO BARE METAL STENTS STILL DESERVE A PLACE IN THE CATH LAB?Drug-eluting stents clearly offer an advantage over BMS with regard to restenosis. Randomized trials and registries have consistently shown the superiority of second-generation DES over BMS regarding clinical and angiographic restenosis (Table 1), with reduced rates of repeat revascularization and ST events, but comparable clinical outcomes (in terms of death and spontaneous MI), as recently shown by the NORSTENT trial.28 Despite this clear advantage, the long-term safety of DES relies on long duration of DAPT, making BMS more appealing in selected clinical settings, in which the patient is unable to complete the recommended duration of DAPT because of nonadherence, need for noncardiac surgery within 1 year from PCI, or an increased risk of bleeding. In these clinical scenarios, the small anticipated benefit to be gained from reduced restenosis may be balanced by the need to withhold the antiplatelet regimen.
It should be stressed that the use of BMS requires careful patient selection, with the exclusion of particular coronary anatomy (bifurcation lesions requiring a 2-stent strategy, long lesions, left main disease, or small vessel diameter < 3mm) and clinical situation (treatment of chronic total occlusion, occlusion of saphenous vein graft, ST-segment elevation MI), in which the use of BMS is considered unadvisable.28,29
The recent availability of polymer-free DES in some countries has further narrowed the area where a BMS may be needed. Metallic stents with a thin biodegradable abluminal polymer layer (or isolated dots) may represent a safer alternative.
CONCLUSION AND FUTURE DIRECTIONSThere is no way we could have foreseen the impact of our work so many years ago. Coronary artery stenting is the treatment of choice for CAD. With the advent of stents, the mechanical contribution to restenosis and acute recoil have been solved, making emergency bypass surgery a thing of the past. A large body of evidence has demonstrated a significant improvement in coronary stent safety and efficacy with device evolution, making second-generation DES the treatment of choice for patients requiring coronary angioplasty. BMS, which have dominated our cath labs for 15 years, remain an option for selected patients, especially those who cannot complete the recommended duration of DAPT.
Now the challenge is to develop the right cocktail of drugs, platforms and coatings to entirely eliminate, not just reduce, thrombosis and restenosis.
CONFLICTS OF INTERESTNone declared.