Renal impairment influences the prognosis of patients with cardiovascular disease and increases cardiovascular risk. Renal dysfunction is a marker of lesions in other parts of the vascular tree and detection facilitates early identification of individuals at high risk of cardiovascular events. In patients with cardiovascular disease, renal function is assessed by measuring albuminuria in a spot urine sample and by estimating the glomerular filtration rate using creatinine-derived predictive formulas or equations. We recommend the Chronic Kidney Disease Epidemiology Collaboration or the Modification of Diet in Renal Disease formulas. The Cockcroft-Gault formula is a possible alternative. The administration of drugs that block the angiotensin-renin system can, on occasion, be associated with acute renal dysfunction or hyperkalemia. We need to know when risk of these complications exists so as to provide the best possible treatment: prevention. Given the growing number of diagnostic and therapeutic procedures in the field of cardiology that use intravenous contrast media, contrast-induced nephrotoxicity represents a significant problem. We should identify the risk factors and patients at greatest risk, and prevent it from appearing.
Keywords
.
Why assess renal impairment in patients with heart disease? Prognostic Relevance of Chronic Kidney DiseaseThe importance of cardiovascular (CV) disease as the first cause of morbidity and mortality in Spain justifies the extraordinary effort invested in researching its etiology and pathogenesis, the prognosis, early detection of markers, and treatment. Hence, in recent years we have become increasingly aware of the role of kidney failure (KF) in the prognosis of CV disease (Table 1).
Table 1. Relationship Between Heart and Kidney: Prognostic Importance.
| • Cardiovascular disease is the first cause of death in the population with CKD |
| • As renal function diminishes, the probability of cardiovascular events increases, even in patients with mildly reduced renal function |
| • Patients with CKD present substantial comorbidity, which favors the development of cardiovascular disease |
| • Some 30%-50% of patients presenting acute coronary syndrome have some degree of renal dysfunction |
| • Up to 50% of patients with heart failure present CKD |
| • Administration of intravenous contrast agents in coronary artery interventions can favor the appearance of renal dysfunction due to nephrotoxicity |
| • Detection of renal dysfunction enables us early identification of those patients at high risk of cardiovascular events in order to improve prognosis through early diagnostic and therapeutic intervention |
CKD, chronic kidney failure.
Patients with reduced renal function are at greater risk of CV complications.1 In those with known heart disease, the risk of death increases as renal function worsens or markers of renal damage (eg, proteinuria) appear. In fact,2 patients with glomerular filtration rate (GFR) <20ml/min/1.73 m2 are at a 6-fold greater risk of death than those with GFR >60ml/min/1.73 m2. This direct relationship between worsening renal function and the appearance of CV events and mortality has been seen in individuals with moderately (or even slightly) reduced renal function, and the risk increases as GFR worsens.1, 2 Many studies and recent meta-analyses3 have shown the presence of chronic kidney disease (CKD)–defined by reduced GFR or presence of renal damage markers (albuminuria/proteinuria)–to be an important predictor of morbidity and mortality.
CKD is a global health problem. We have seen a progressive increase in patients starting renal replacement therapy–currently 5% to 8% per year4–and, moreover, this constitutes an economic issue of some consequence. In Spain,5 6.8%2 of the population presents reduced GFR of <60ml/min/1.73 m2. Essentially, the factors underlying the increased prevalence of CKD are the progressive aging of the population in the developed world and the increased prevalence of diabetes mellitus and of high blood pressure, most of which are present in patients with heart disease.
Furthermore, patients with CKD have substantial comorbidity, which favors the development of the CV disease that is the principle cause of death in these patients, affecting approximately half of all CKD cases.4.
Chronic Kidney Disease and Heart DiseaseThe prevalence of CKD among patients with heart disease is extremely high and influences prognosis.
High Prevalence of Chronic Kidney Disease in Patients With Ischemic Heart DiseaseSome 30% to 50% of patients with acute coronary syndrome have some degree of renal dysfunction2 and its presence has clear CV prognostic significance.2, 6, 7.
After coronary revascularization, mortality among patients with KF (GFR <60ml/min/1.73 m2) is similar to that of patients with previous myocardial infarction.8 This increase in KF-related mortality is not due to a higher restenosis rate8 and may be explained by greater presence of CV risk factors–especially the more severe and diffuse lesions of the coronary tree and longer clinical course of heart failure.8, 9.
Among patients with heart disease undergoing surgery–especially bypass surgery–presence of KF represents a 3-fold increase in risk of renal dysfunction as a postoperative complication; they are much more likely to need dialysis than would patients with normal renal function. Even a slight deterioration in renal function has been associated with a significant increase in morbidity and mortality.10.
High Prevalence of Chronic Kidney Disease in Patients With Heart FailureUp to 50% of patients with heart failure present CKD. In a prospective multicenter study with more than 1000 patients hospitalized for heart failure, prevalence of renal dysfunction was 27% and in most cases it occurred within the first 3 days of hospitalization. The independent risk factors associated with greater risk of heart failure were a history of heart failure, diabetes mellitus, serum creatinine >1.5mg/dl, and blood pressure >160mmHg.11 One study conducted in Spain has shown that prevalence of KF in patients with heart failure was 43.3% in those with preserved systolic function (left ventricular ejection fraction ≥50%) and 41.8% in those with reduced systolic function (left ventricular ejection fraction <50%).12.
In contrast, 40% of patients with KF have heart failure and this prevalence increases as renal function worsens.
Cardiorenal SyndromeRecently, the term “cardiorenal syndrome” has been coined to describe the relationship between the CV and renal systems because many patients present different degrees of dysfunction in one organ or the other.
The cardiorenal syndrome is a pathophysiologic condition of heart and kidney–the primary dysfunction of one organ, whether acute or chronic, gives rise to secondary dysfunction or lesions in the other–which shows the negative effects reduced renal function has on the heart.
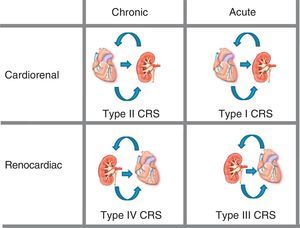
Five types of cardiorenal syndrome have been defined13 (Figure 1):
• Type I (acute cardiorenal syndrome): acute worsening of heart function producing acute renal damage.
• Type II (chronic cardiorenal syndrome): chronic abnormalities in heart function (eg, congestive heart failure) that cause progressive, permanent CKD.
• Type III (acute renocardiac syndrome): acute worsening in renal function (eg, acute ischemia or glomerulonephritis) that causes acute heart injury (heart failure, arrhythmia, ischemia).
• Type IV (chronic renocardiac syndrome): CKD (eg, due to glomerular disease or nephroangiosclerosis) contributing to reduced heart function, cardiac hypertrophy, and/or increased risk of a CV event.
• Type V (secondary cardiorenal syndrome): a systemic condition (eg, diabetes mellitus, sepsis) causing simultaneous renal and cardiac dysfunctions.
Figure 1. Cardiorenal syndrome: kidney-heart bi-directionality. CRS, cardiorenal syndrome. Modified with permission from Ronco et al. 13
Why Assess Renal Function in Patients With Heart Disease?The purpose of detecting renal dysfunction is early identification of patients at high risk of CV events so as to improve their prognosis through early diagnostic intervention and treatment. The kidney is a specialized endothelium and any lesion it incurs may be a marker of other lesions elsewhere in the vascular tree, especially in the heart. As well as diagnosing and assessing CV risk, detecting and estimating the level of renal injury enables us to monitor interventions such as adjusting drug regimens (digoxin, some beta blockers) and detect patients at risk of nephrotoxicity (after administering contrast media or, in certain circumstances, nonsteroid anti-inflammatory drugs, etc.).
Assessing renal impairment in patients with cardiovascular diseaseIn patients with CV disease, renal dysfunction is assessed by measuring albuminuria and estimating GFR with serum creatinine-derived formulas. The presence of both (albuminuria and reduced GFR) has a synergistic effect on CV risk prediction.14, 15 In European Society of Hypertension and European Cardiology Society guidelines, estimated GFR <60ml/min/1.73 m2 or above-normal urinary albumin excretion indicate subclinical organic lesion.16 Examination of urine sediment completes our screening of the patient with CV disease as it helps rule out non-CV-risk-related illness.
The simultaneous appearance of albuminuria and reduced GFR has proved an independent predictor of CV mortality in the general population.17 These data have raised the question of whether future clinical practice guidelines should add the suffix (P) to indicate presence or absence of albuminuria/proteinuria), along with the CKD stage, to improve patient CV-risk stratification. In patients with the same CKD stage, presence of albuminuria would be a differentiating factor for prognosis.17.
Definition of Chronic Kidney DiseaseTo clarify the definition and classification of CKD, the US National Kidney Foundation published directives to unify criteria defining renal function status (Kidney Disease Outcomes Quality Initiative, Guidelines K/DOQI).18 This classification is based on calculating estimated GFR with the Modification of Diet in Renal Disease (MDRD) formula, rather than creatinine concentration. It enables us to analyze many studies simply, and even retrospectively, which has helped us learn more about the impact of CKD on CV prognosis (Table 2).
Table 2. Stages of Chronic Kidney Disease Estimated by Glomerular Filtration Rate. Kidney Disease Outcomes Quality Initiative Classification.
| Stage | Description | eGFR, mil/min/1.73 m2 |
| Without CKD | At-risk patients | >90, with risk factor |
| 1 | Renal lesion * with normal or increased eGFR | >90 |
| 2 | Slight eGFR decline | 60-89 |
| 3 | Moderate eGFR decline | 30-59 |
| 4 | Substantial eGFR decline | 15-29 |
| 5 | Terminal kidney failure | <15 or dialysis |
CKD, chronic kidney failure; eGFR, estimated glomerular filtration rate.
* US National Kidney Foundation definition of renal lesion: histopathologic abnormalities or markers of renal lesion, including abnormalities in blood and urine or diagnostic imaging studies.Adapted with permission from the US National Kidney Foundation. 18
The K/DOQI definition of CKD includes:
• Renal injury of ≥3 months, defined by renal lesion diagnosed directly (histopathologic abnormalities in renal biopsy) or indirectly by markers such as albuminuria or proteinuria, by urine sediment abnormalities, or in imaging studies.
• GFR <60ml/min/1.73 m2 during >3 months with or without renal injury.
In other words, the concept of CKD should consider both dimensions: reduced GFR and presence of albuminuria. Any patient with persistent albuminuria for more than 3 months is considered to have CKD, even though they may present GFR >60ml/min/1.73 m2. The term chronic KF (as opposed to CKD) is defined as progressive (≥3 months), irreversible loss of renal function; the level of impairment is determined by GFR <60ml/min/1.73 m2.
Measuring Albuminuria Albuminuria and Cardiovascular RiskAlbuminuria means abnormalities exist in the glomerular filtration barrier (basal membrane) and may reflect generalized vascular dysfunction.19 It is a CV risk factor both in patients with and without diabetes.19, 20 Its value, which is continuous, is also considered prognostic in patients with albuminuria within the “normal” range.20 Reduced albuminuria is associated with a reduction in CV and renal events.21 Albuminuria has been associated with greater risk of heart failure and coronary disease–a risk that grows as albuminuria increases.22, 23.
Definition of AlbuminuriaAlbuminuria is defined as urinary albumin elimination >30mg/24h, equivalent to 20μg/min in urine collected over a specific period or 30mg/g creatinine in a spot sample (Table 3). Higher values are associated with greater CV and renal risk and the presence of subclinical organic renal lesion.16 Although a quantitative parameter has been used to define albuminuria (>30mg/g), it should be considered a continuous variable; CV risk increases in line with urinary albumin excretion from as little as 8 to 10mg/day.24 Therefore, it is considered that “urinary albumin excretion” is the most appropriate way to define it and that, even within the normal range, increased urinary albumin excretion is associated with worse cardiovascular and renal prognosis.24 Urinary albumin excretion of >300mg/day in a sample or 300mg/day in 24-h urine is considered proteinuria and indicates established nephropathy.
Table 3. Albuminuria in the Detection of Renal Lesions.
| • Method of measurement (of choice): albumin/creatinine ratio (mg/g creatinine) |
| • Definition of albuminuria: >30 mg/g in spot urine sample |
| • Given measurement variability, 2 out of 3 positive measures are needed over 3-6 months to consider it pathologic |
| • Valid samples: first morning, mid-morning and mid-afternoon urine samples |
| • Situations that increase albuminuria: intense physical exercise, fever, infection, heart failure, hyperglycemic decompensation |
| • False positives: hematuria, pyuria, highly concentrated urine |
| • Less accurate albumin/creatinine ratio in extreme values of creatinine: overestimated in reduced muscle mass and underestimated in muscular patients |
For more than 20 years, semiquantitative diagnostic tests to measure albuminuria have been available to physicians in the form of reactive strips (Micral-Test and Clinitek) with a sensitivity and specificity of 80% to 97% and 33% to 80%, respectively. Given the low specificity and high false positive and false negative rates, these tests are only used when standard immunological techniques (immunonephelometry, immunoturbidimetry and radioimmunoanalysis) are unavailable.
24-h urine samplingMeasuring proteinuria or albuminuria in 24-h urine has been considered the reference pattern (assuming collection is conducted correctly) but also has been associated with important errors derived from incomplete urine collection; therefore, it should not be used in screening.
Albumin/creatinine ration in a urine sampleMeasuring albuminuria by analyzing the albumin/creatinine ratio in a spot sample is recommended when screening patients with high blood pressure or diabetes mellitus, and to determine CV or renal risk.24, 25, 26 This avoids 24-h or (specific) timed urine collection, first–morning-voided collections are not essential, results correlate with those obtained from 24-h urine collection, and it is inexpensive (1-2 €/nephelometry assessment) and can be repeated to confirm the presence or absence of albuminuria (Table 3). Nonetheless, first-morning-voided urine is preferable.
Factors That Modify Albumin Elimination. LimitationsGiven the variability of urinary albumin excretion, 2 to 3 positive measurements over 3 to 6 months are needed to establish pathology. Although recommendations on screening frequency do not exist, except in diabetes mellitus, measuring albuminuria should be part of initial assessment and CV risk stratification in any patient with high blood pressure, diabetes mellitus, or CV disease. If albuminuria values are pathologic, testing should be repeated to confirm (2 out of 3 samples).26 Later, annual or more frequent regular assessment is recommended, depending on results and on the therapeutic objectives established. In patients with lower than normal or low-to-normal (<15mg/g) levels, routine screening can be every 3 to 5 years.
Albuminuria levels can be influenced–and may be modified–by other clinical conditions. Intense physical exercise, active infection, fever, hyperglycemic decompensation, or heart failure can increase albuminuria values. Other situations–highly concentrated urine hematuria or pyuria–produce elevated values due to “false positives.”
The accuracy of albumin/creatinine ratio measurement falls if creatinine excretion values differ from those expected (the value of the denominator in the equation). This should be taken into account when values approach the limits of the range. For example, the albumin/creatinine ratio can overestimate albumin in patients with reduced muscle mass. In contrast, in very muscular patients or blacks (African-Americans) it can be underestimated27 (Table 3).
Although the mechanism by which albuminuria is associated with greater CV risk is not fully understood, through albuminuria the kidney provides very precise, early data on the status of the rest of the vascular tree. Hence, this should be part of CV risk assessment and stratification.
Renal Function Assessment in Patients With Cardiovascular Disease Glomerular Filtration Rate and Creatinine ClearanceGlomerular filtration measures the rate at which liquid leaves the glomerular capillaries for the Bowman capsule and reflects water and small solute filtration. Renal glomeruli filter 125ml of liquid/min (180 l/day) (20% of cardiac output). This is termed GFR and is the total filtration of each of some 2 million functioning nephrons. Reduced GFR indicates KF.
Normal GFR varies as a function of age, sex, and body size. In young adults, it ranges from 120 to 130ml/min/1.73 m2 (or 180 l/day/1.73 m2). The result is corrected for body surface and expressed as ml/min/1.73 m2. From age >40 years, GFR falls at approximately 10ml/min/1.73 m2/decade. The gold standard to determine GFR is to calculate clearance of inulin and of radioactive isotopes (51Cr-EDTA, 99Tc-DTPA, 125I-iothalamate), but it cannot be applied in daily clinical practice.
Creatinine clearance is one way of measuring GFR. The clearance of a substance is the quantity of that same substance extracted from the plasma during a specific time period. Hence, creatinine clearance is the quantity of creatinine extracted from plasma in 24h. When renal function is determined using 24-h urine, it is measured by creatinine clearance because this calculation uses urinary creatinine and plasma creatinine (concept of clearance). Similarly, the Cockcroft-Gault formula28 gives estimated creatinine clearance expressed in ml/min because the formula uses two 24-h urine samples for creatinine clearance as the gold standard. It is not corrected for body surface. In contrast, estimating renal function with creatinine-derived formulas–such as MDRD29 or CKD-EPI30–gives estimated GFR expressed in ml/min/1.73 m2 because this formula uses 125I-iothalamate, which measures GFR, and the result is adjusted for body surface.
Therefore, renal function can be measured through creatinine clearance using 24-h urine or estimated through creatinine-derived predictive equations or formulas: the MDRD or CKD-EPI formulas, which estimate GFR, and the Cockcroft-Gault formula, which estimates creatinine clearance.
Plasma Creatinine as a Marker of Renal FunctionSerum creatinine concentration, because of its simplicity and rapidity, has been used to measure renal function. In daily clinical practice it has been standard practice to interpret renal function values on the basis of serum creatinine. However, creatinine concentration is affected by a variety of factors (muscle mass, sex, race, diet) in addition to those related to creatinine filtration itself, such as tubular secretion and extra-renal production and excretion.31.
In initial stages of KF, when GFR is practically normal, reduction entails only a slight increase in plasma creatinine because in this situation the proximal tubular secretion of creatinine rises. Consequently, with already-reduced GFR the plasma creatinine is found to be within the normal range; therefore, normal or nearly normal plasma creatinine does not necessarily imply GFR has been maintained. When GFR falls to ±50%, serum creatinine barely increases (eg, 1.3mg/dl); when it reaches 1.5mg/dl, renal function has worsened by two-thirds with respect to baseline. Serum creatinine may be within the normal range and GFR may be reduced (<60ml/min/1.73 m2). This situation is termed hidden kidney disease.
Estimating Renal Function/Glomerular Filtration RateAs the creatinine figure is not the best indicator of renal function, we should look for better markers. Hence, we resort to estimating GFR or creatinine clearance.
24-h urine creatinine clearanceMeasuring creatinine clearance with 24-h urine is inconvenient because tubular secretion may vary, collection may be incomplete, and the method is uncomfortable for the patient who has to carry a urine bottle for 24h. Moreover, except in certain circumstances, it does not improve on estimated GFR as calculated using the equations. Therefore, it is not used as the standard method or in screening.
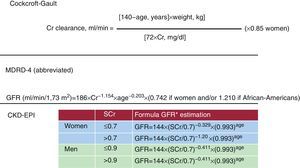
Measuring Glomerular Filtration Rate With Creatinine-Derived FormulasCurrently, in daily clinical practice, GFR estimation through formulas is the best available test of renal function in patients with CV disease. We use predictive equations that include creatinine, sex, age, and patient weight.28, 29, 30, 31, 32, 33 Figure 2 shows the formulas recommended to calculate estimated creatinine clearance.
• The Cockcroft-Gault formula for creatinine clearance. To calculate the Cockcroft-Gault formula,28 we need to know patient body weight. The result can be corrected for body surface of 1.73 m2 using another formula. The disadvantage of this formula is that body weight data is not always recorded on the laboratory sheet. Typically, the formula has been used to adjust drug regimens to renal function. However, the approximately 10% to 15% overestimation of renal function with this formula, and the need to know patient weight, somewhat limit regular use.
• MDRD equation. The abbreviated MDRD study equation29 only requires data on patient age and sex; the result self-corrects for body surface. The abbreviated MDRD equation29 is considered more reliable than the Cockcroft-Gault formula,28 especially when GFR is <60ml/min/1.73 m2. It is easier to calculate, and therefore its use in daily practice is encouraged.32, 33 However, with normal or nearly normal renal function, calculating GFR with the MDRD equation can underestimate renal function, especially in women. Consequently, it is recommended that values >60ml/min/1.73 m2 be reported as >60ml/min/1.73 m2, and that numeric values calculated by the estimation equation are not given. Thus, the Cockcroft-Gault formula may be quite useful in this situation (bearing in mind that it overestimates renal function by ±10%).
• CKD-EPI equation. Recently a modified MDRD formula has been published: the CKD-EPI formula.30 This reduces the bias or underestimation of the MDRD formula, above all in GFR >60ml/min/1.73 m2. Therefore, we propose that the CKD-EPI formula should replace the MDRD formula in daily clinical practice. Although the perfect formula has yet to be described, the CKD-EPI formula may currently be the least imperfect means of estimating GFR.
Figure 2. Different formulas for creatinine clearance and glomerular filtration rate estimations. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Cr, creatinine; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine (mg/dl). *If blacks, rather than 144 is 166 for women and 163 for males.
These formulas, despite their mathematical complexity, can be calculated on many websites (eg, Spanish Society of Nephrology: www.senefro.org). Laboratories are recommended to provide it automatically in their analyses when patient age and sex data are available.
The Spanish Society of Nephrology and the Spanish Society of Family and Community Medicine consensus document on CKD makes some recommendations on renal function assessment. These include situations in which 24-h urine should be used33 (Table 4).
Table 4. Principle Recommendations on Renal Function Assessment.
| Serum creatinine measurement should not be used as the only parameter to evaluate renal function. Equation-based estimated GFR is the best index available in daily clinical practice to assess renal function |
| To estimate GFR, we first recommend the CKD-EPI or MDRD study formulas. As an alternative the Cockcroft-Gault formula can be used |
| Measurement of creatinine clearance using 24-h urine samples does not improve, except in specific circumstances, equation-derived estimated GFR |
| The circumstances in which equations are not useful are: |
| • Extreme body weight: body mass index <19 or >35 kg/m2 |
| • Significant muscle mass abnormality (amputations, loss of muscle mass, muscle disease, or paralysis) |
| • Acute kidney failure, pregnancy, severe hepatopathy, generalized edema, or ascitis |
| In these cases, we recommend the use of other methods of GFR estimation, such as conventional (24 h urine) creatinine clearance |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
We include those situations in which 24-h urine should be used to estimate renal function.
Modified with permission from Alcázar et al. 33
As the predictive formulas have been calculated with specific population groups and serum creatinine-derived formulas, the equations to estimate GFR are inadequate in the following circumstances:
• Extreme body weight: body mass index <19 or >35 kg/m2.
• Significant muscle mass abnormality (amputation, loss of muscle mass, muscular illnesses or paralysis).
In the special, relatively infrequent, clinical situations when estimating GFR by formula is inappropriate, estimated creatinine clearance with 24-h urine should be used.33.
Prevention of acute renal dysfunction and hyperkalemia. precautions and limitations of renin-angiotensin-aldosterone blockersBlocking the renin-angiotensin-aldosterone system (RAAS) plays an essential role in human CV pathology and forms part of the treatment approach in patients with CKD (albuminuria or reduced GFR) and in those with heart disease (ischemic heart disease, heart failure, and atrial fibrillation).
Treatment with RAAS blockers (angiotensin converting enzyme inhibitors [ACEI], angiotensin receptor blockers [ARB] or direct renin inhibitors) does not usually cause renal dysfunction or hyperkalemia in patients with normal renal function. These complications can be observed in patients with high CV risk and generalized atheromatous disease such as, of course, renal atheromatosis and/or abnormal renal function.
Acute renal dysfunction, hypotension, and/or hyperkalemia occur because of a reduction in systemic blood pressure and intraglomerular pressure as the efferent arteriole dilates, with the consequent reduction in GFR. Thus, it is important to recognize the risk situations when these complications can present in order to provide the best possible treatment, prevention, and monitoring for patients at risk of these complications.
Autoregulation of Renal Blood FlowIn the healthy kidney an autoregulatory process keeps renal blood flow and intraglomerular capillary pressure constant despite systemic blood pressure fluctuations between 80 and 170mmHg. When systemic blood pressure falls, glomerular pressure holds steady because of the angiotensin II, which causes vasoconstriction of the efferent arteriole (postglomerular arteriole).34 In elderly patients, patients with chronic hypertension, and those with diabetes, and in advanced atheromatosis, the autoregulatory capacity is partially lost. In other words, the patient has little capacity to compensate for the lower vasoconstrictive response of the efferent arteriole because they have atheromatosis (with hyalinosis or hypertrophy of the intima). Therefore, these patients more frequently present acute renal dysfunction following the aforementioned drug treatments. This renal deterioration is hemodynamic (and reversible in most cases); it is not the result of structural renal injury, and reflects the fact that blood pressure has fallen below renal autoregulation limits.
In daily clinical practice, when a worsening of renal function as the response to initiating a RAAS drug regimen is detected, some physicians first withdraw the drug or reduce the dosage. Both of these strategies lead to increased blood pressure and glomerular pressure, and the creatinine concentration returns to its original level. This approach is not enough to preserve mid- or long-term renal function or compensate for the effects of angiotensin on the heart.
Following RAAS blocking treatment, if serum creatinine increases slightly (always <20%-30% of the baseline figure) in the context of adequate control of blood pressure values, we can consider glomerular pressure to have fallen satisfactorily. This is of benefit in preventing kidney disease progression35, 36, 37 (both for albuminuria and preventing reduced GFR) and other heart conditions (heart failure, ischemic heart disease).38, 39.
When Is Worsening Renal Function Following Renin-Angiotensin-Aldosterone System Blockers Beneficial and When Is It a Danger?Generally, this is considered dangerous when accompanied by hyperkalemia (K >5.5 mEq/l) due to its cardiac consequences, and when worsening renal function is >30% over baseline because it can favor more severe KF, with other complications that sometimes lead to the need for dialysis. In both cases, the drug should be withdrawn and we should establish whether the situation favors renal deterioration, in order to treat it if possible or learn more about it so we are aware of the limitations we face when administering specific treatments.
Renal Dysfunction and/or Hyperkalemia After Administering Renin-Angiotensin-Aldosterone System BlockersAfter initiating RAAS blocker treatment, precautionary measures are only needed to avoid acute renal dysfunction, hypotension, and/or hyperkalemia in patients with risk factors for these conditions; they are not needed in all patients administered these drugs.
Hypotension and Renal DysfunctionThis can appear as weakness, hypotension, syncope, or dizziness in the baseline stage or on assuming an upright posture. In the ONTARGET study,40 this secondary effect occurred in 1.7% of patients receiving ramipril or telmisartan and in 4.7% of those receiving the combination (P<.001). Hypotension, especially after the first dose, usually occurs in highly hypovolemic patients presenting vomiting or diarrhea or receiving intensive diuretic treatment. Occasionally, the patient is stable and receives the RAAS blocker, but hypotension can occur if diuretic treatment is increased or they present any of the previously mentioned complications. It frequently occurs in patients who have been hospitalized for heart failure. On discharge, they receive moderate/high doses of distal diuretics (antialdosterone drugs) which should be adjusted, generally reduced, during out-patient follow-up according to the patient's clinical response. If not, the patient can receive excessive diuretic treatment and present hypotension, which favors hyperkalemia and/or renal dysfunction.
Both renal ischemia due to atheromatosis and, essentially, the detection of a low sodium concentration due to the macula densa in stages of depletion (excessive diuretic treatment, excessively low-sodium diets, diarrhea, vomiting) induce substantial RAAS activation to maintain blood pressure and renal perfusion. To keep GFR at an adequate level, angiotensin II increases the efferent arteriole (postglomerular vasoconstriction) resistance. If we administer a RAAS blocker (ACEI, ARB, or direct renin inhibitor), we block the mechanism that maintains blood pressure and intraglomerular pressure falls substantially, as does secondary GFR; creatinine increases, generally by >30%.
When the patient presents hypotension after receiving these drugs, we need to investigate possible causes.35 The most frequent causes are listed in Table 5. We also have to consider the presence of significant bilateral stenosis of the renal artery as a possible cause. In patients who present unilateral renal artery stenosis that is not susceptible to angioplasty, RAAS blockers can be administered. In these patients, worsening GFR is barely appreciable, as is a fall in systemic blood pressure, because GFR increases in the contralateral kidney to compensate.41.
Table 5. Risk Factors Indicating Worsening Renal Function Following Treatment With Renin-Angiotensin-Aldosterone System Blockers.
| Advanced renal insufficiency |
| Bilateral renal artery stenosis |
| Reduced blood volume: |
| • Hypotension |
| • Volume depletion (diuretics, vomiting, diarrhea) |
| • Use of non-steroid anti-inflammatory drugs |
| • Sepsis/vasodilatation |
The risk of hyperkalemia in patients receiving RAAS blockers is ±3.3%40 but this increases as other risk factors present and because of their combination with other drugs, especially distal diuretics (antialdosterones) and nonsteroid anti-inflammatory drugs42 (Table 6). Increased potassium, within the normal range (eg, 4.5-5 mEq/l), does not require drug withdrawal or dose adjustment, as RAAS blocking associates with improved CV prognosis because it provides nephro-21, 43 and cardioprotection.38, 39 These drugs cannot be administered if hyperkalemia presents after the trigger mechanisms have been corrected. It is always best to balance the benefits and risks of these treatments.
Table 6. Factors Favoring Hyperkalemia.
| Advanced age |
| Kidney failure, hidden kidney failure |
| Diabetes mellitus with elevated baseline potassium |
| Volume depletion |
| Elevated baseline potassium |
| Use of potassium salts as supplement (dietary salt) |
| Previous treatment with: |
| • ACEI/ARB, NSAI, spironolactone, amiloride, beta blockers |
| • High doses of the aforementioned treatments |
| • Combination of several of the above treatments |
| Tissue hypoperfusion (generalized atheromatosis): |
| • Stroke, ischemic heart disease, intermittent claudication, intestinal angina, kidney failure, smoking |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; NSAI, nonsteroid anti-inflammatory drugs.
Modified with permission from Palmer et al. 42
Low incidence of hyperkalemia in clinical trials is attributed to the fact that patients are low risk and those with KF are frequently excluded; moreover, they are closely monitored. Furthermore, the age of the patients included in studies should be taken into account. The mean age of the CHARM study39 was 65 years. Even further removed from the context of daily clinical practice is the situation in which heart failure treatment with distal diuretics is analyzed. The mean age in studies using spironolactone or eplerenone in heart failure associated with other clinical conditions (RALES44, EPHESUS45 and EMPHASIS46) was 65, 64, and 68 years, respectively; mean creatinine was 1.1mg/dl. Extrapolating the results of these studies in a selected population to patients in daily clinical practice favors severe hyperkalemia, as the percentage of elderly patients attending clinics is very high; many are octogenarian or nonagenarian, with more or less reduced GFR.
How Can We Minimize the Risk of Acute Renal Dysfunction and/or Hyperkalemia in Patients Receiving Renin-Angiotensin-Aldosterone System Blockers?We propose the following approaches:
• Identify and correct factors associated with the appearance of hyperkalemia and/or renal dysfunction (Table 5, Table 6).
• Estimate risk of hyperkalemia, especially due to advanced age, substantial depletion, and concomitant drugs. If any potentially reversible factor exists, it should be corrected prior to ACEI or ARB administration.
• Renal function must be measured (MDRD, CKD-EPI or Cockroft-Gault formulas). Patients with normal renal function rarely present hyperkalemia. As renal function is reduced, the risk of hyperkalemia increases.
• In patients with normal renal function and without risk factors, full doses of ACEI or ARB can be administered. In this situation, no analytical checks are needed after drug regimen initiation and simultaneous use of more than one drug group can be considered.
• In patients with risk factors or KF but who may benefit from RAAS blockers for nephro- or cardioprotection, or any other indication, we recommend initially low doses of ACEI or ARB followed by a gradual increase in dosage. If patients have several risk factors (Table 6), we advise monitoring creatinine and potassium at 7 to 10 days after initiating treatment, particularly if we aim to achieve a moderate fall in blood pressure following the first dose. In this case, we should seek to rule out bilateral renal artery stenosis and other causes described in the risk factors, especially depletion. If RAAS blocker dosage is increased, potassium and creatinine monitoring should again be considered.
• In stable patients receiving chronic treatments with ACEI or ARB, hyperkalemia, hypotension, or acute renal dysfunction can present in the context of excessive diuretic treatment, acute diarrhea, vomiting, or any other cause of depletion. In these cases, ACEI or ARB should be temporarily withdrawn until the patient recovers from hypotension; any factors favoring this situation should be corrected. Later, if indicated, RAAS blocker treatment will be reinitiated, progressively if possible.42.
Other issues to bear in mind are:
• Prescribe antialdosterone drugs carefully, bearing in mind patient age and renal function and clearly establishing the clinical indication.
• Do not administer potassium or salt (potassium chloride) dietary supplements to patients at risk of hyperkalemia or with estimated GFR <30ml/min/1.73 m2, despite administering high doses of loop-acting diuretics. This level of GFR is considered the greatest risk of hyperkalemia and/or acute renal dysfunction. If RAAS blockers are used, potassium and creatinine should be monitored frequently (eg, every 2-3 months minimum).
• Weight is a good indicator of the patient's state of hydration. Short-term weight reduction in patients receiving diuretic drugs can indicate an excessive dosage and, often, this should be reduced unless the clinical condition indicates the contrary.
• Inform the patient of any possible interaction with other medication (especially avoid nonsteroid anti-inflammatory drugs).
• Restrict dietary potassium.
• Use loop-acting diuretics (furosemide or torasemide) if not previously administered (thiazides have less effect on KF).
• Add potassium capturing resins (calcium polystyrene sulfonate) (Resincalcio®, Sorbisterit®), one 15-gram dose after lunch and dinner, bearing in mind that the taste is unpleasant and that it causes a degree of constipation (depending on the dose). We advise administering these together with an osmotic laxative which also favors potassium loss via the intestine.
Metabolic acidosis needs correcting (sodium bicarbonate) if it occurs, especially in patients with KF.
If potassium remains high (>5.5 mEq/l) despite these measures, and having withdrawn suspect drugs, the patient can be assumed to have hyporeninemic hypoaldosteronism. In this case, RAAS blockers cannot be administered. Diuretics, calcium antagonists or even beta blockers can be used (they do have a renin inhibition effect but it is less powerful than that of ACEI and ARB).
Contrast- and drug-induced nephrotoxicity Contrast-Induced NephropathyThe progressive increase in patients undergoing diagnostic and therapeutic procedures with intravenous contrast media makes contrast-induced nephrotoxicity a highly relevant issue, especially in the field of cardiology.
Definition and Patients at RiskContrast-induced nephropathy (CIN) is defined as worsening renal function that translates into a 25% relative increase or a 0.5mg/dl absolute increase in creatinine with respect to baseline data, occurring in the first 3 days after contrast media is administered and not due to any other mechanism.
Despite being highly prevalent (it is the third cause of acute renal failure in hospitalized patients), the pathophysiology of CIN is not well defined. Many studies have concluded that CIN is caused by a combination of mechanisms: the direct effect of the contrast media, which reduces renal perfusion, rheologic abnormalities in the renal tubule due to increased blood viscosity that the contrast medium causes, and the direct toxic effect on the tubular cells.47.
Of all the procedures in which intravenous contrast media are used, coronary artery interventions (angiography and diagnostic or therapeutic percutaneous coronary interventions) are those most associated with CIN. In prospective studies, CIN incidence rises to 3.3%.48 Of these patients, the subgroup that has also had a myocardial infarction and required primary angioplasty has the greatest propensity to CIN, with 19% incidence.49.
In 80% of CIN cases, creatinine elevation occurs in the first 24h. When creatinine increase is <0.5mg/dl, the probability of CIN is low. In contrast, most patients whose clinical course leads to established acute renal failure had presented increased creatinine during the first day.50.
Not all patients have the same risk of CIN when administered intravenous contrast media. Table 7 describes the most important CIN risk factors. Detecting and modifying these as far as possible prior to administering contrast media is essential to avoid CIN appearing.51.
Table 7. Modifiable and Nonmodifiable Risk Factors Associated With Contrast-Induced Nephropathy.
| Nonmodifiable risk factors | Modifiable risk factors |
| Age | Volume of contrast administered |
| Diabetes mellitus | Hypotension |
| Renal insufficiency (glomerular filtration rate <60 ml/min) | Anemia |
| Advanced heart failure | Dehydration |
| Low left ventricular ejection fraction | Use of drugs: ACEI, ARB, diuretics, NSAI |
| Acute myocardial infarction | Hypoalbuminemia |
| Cardiogenic shock | Use of intra-aortic balloon |
| Kidney transplantation | Use of nephrotoxic antibiotics |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; NSAI, nonsteroid anti-inflammatory drugs.
The most important way to prevent CIN is to detect those patients at risk of having it (Table 7). Administering prophylactic treatment prior to contrast media can avoid complications and reduce hospital stay. N-acetylcysteine (NAC) prophylaxis has been used for this, and above all, correct hydration.
Correct HydrationFluids therapy is the cornerstone of CIN prevention. Many randomized observational studies have demonstrated its effectiveness. Fluids protocols differ but all studies coincide in that combined intravenous fluids therapy and oral hydration is the best means of preventing CIN, especially in mid-high risk patients. The recommended dose–always assuming logistics permit–is physiologic saline solution (0.9%), initiated 12h prior to the procedure at 1ml/kg/h and maintained until 24h postprocedure. Moreover, we recommend oral hydration, with an intake of at least 500ml liquid (water, tea, etc.) on the day of the test and up to 2500ml during the following 24h.52.
Some isolated studies have shown that intravenous administration of sodium bicarbonate as part of fluids therapy can be beneficial in preventing CIN, as it produces alkalinization in the renal tubule, reducing the formation of free radicals.53.
Pharmacologic ProphylaxisIn recent years, many studies have tried to identify drugs that might protect against CIN. Many, like calcium antagonists, mannitol, theophylline, or fenoldopam, have not proven effective; however, NAC has obtained benefits in several studies.54, 55NAC is a powerful antioxidant that eliminates a wide range of free radicals and is capable of preventing CIN, improving renal hemodynamics, and preventing the direct damage of oxidative stress. Several small, randomized prospective studies have concluded that NAC administration with adequate hydration significantly reduces CIN in high-risk patients; however, other studies find no benefit in adding NAC to standard hydration.56, 57, 58.
Some meta-analyses show inconclusive data due to the heterogeneity of the studies included and indicate NAC only seems to prevent CIN in patients with baseline creatinine <1.9mg/dl or receiving >140ml contrast media.59 Other studies have tried to establish the efficient dosage of NAC and conclude its protective effect against CIN depends on the dose since nephroprotection is greater with 1200mg oral doses prior to the procedure and 1200mg every 12h for 2 days postprocedure.58, 60.
All studies agree that NAC is safe and inexpensive; therefore, together with fluids therapy it is recommended for all patients in order to prevent CIN, especially in those at high risk.60, 61 When possible, NAC administration should be intravenous as its bioavailability is greater.
Detecting Potentially Prejudicial Concomitant DrugsSeveral drug groups can increase the probability of CIN due to their effect on renal integrity.
Drugs affecting renal hemodynamics:
• Nonsteroid anti-inflammatory drugs and cyclooxygenase 2 inhibitors. These drugs should be withdrawn before administering contrast media due to the increased risk of renal failure since they affect the glomerular hemodynamic mechanism.62.
• Antihypertensive treatment. Blood pressure should be held steady before the procedure, as the patient will receive intense fluids therapy. Avoid blood pressure levels 20 to 30mmHg lower than normal and, as far as possible, do not administer contrast media if blood pressure is unacceptably low. ACEIs and ARBs are most frequently associated with CIN, especially in patients with depletion.35, 46.
• Dopamine. No trial has demonstrated its benefits in CIN prevention.
Drugs that cause tubular toxicity:
• Diuretics: often unavoidable for the patient; the clinician should know that although non-toxic, diuretics increase the risk of hypovolemia. Programmed withdrawal should take place at least 1 day before the procedure.63.
• Aminoglycosides: should be avoided because they are known to cause medullary and interstitial renal lesions.
• Tacrolimus and cyclosporine A: usually essential for the patient, hence radiologists should be informed so they can try to reduce the volume of contrast administered.
Drugs that are potentially toxic following administration of contrast material:
• Metformin: an oral antidiabetic drug that, when it accumulates in the organism due to reduced GFR and especially in situations of hypoperfusion, can be associated with severe lactic acidosis. In patients who will receive intravenous contrast media, metformin use is controversial. The US Food and Drug Administration recommends withdrawing metformin on the day of the test and reintroducing it in 2 to 3 days.62 However, a recent systematic review of guidelines recognizes that insufficient information is currently available to establish metformin withdrawal in patients with previously normal renal function and who are to receive a “moderate” quantity of contrast media.64.
The toxic effect of the contrast medium in the kidney begins within a few minutes of exposure; in fact, the first markers of tubular damage appear in urine in the first hours. However, serum creatinine increases more slowly, beginning on the first day and peaking a maximum of 3 to 5 days after contrast administration; it falls back towards its baseline level in 1 to 3 weeks.65.
In patients with CIN, renal function should be monitored during follow-up until baseline creatinine levels have been recovered.
Gadolinium Use in Patients With Chronic Kidney DiseaseGadolinium is used as contrast a medium to improve results in magnetic resonance images and had been proposed as a potentially less nephrotoxic alternative. Although it is a nephrotoxic contrast medium, its use in patients with KF can be associated with a systemic disturbance known as nephrogenic systemic fibrosis. This is a progressive alteration causing skin fibrosis and can also affect subcutaneous tissue and internal organs. It usually evolves slowly, with fatal consequences, and has no specific treatment. Hence, gadolinium use is not recommended66 in patients with GFR <30ml/min/1.73 m2 and we suggest evaluating the risks and benefits of its use in patients with GFR 30 to 60ml/min/1.73 m2.
Conflicts of interestNone declared.
Received 11 August 2011
Accepted 22 August 2011
Corresponding author: Servicio de Nefrología, Hospital Universitario Dr. Peset, Avda. Gaspar Aguilar 90, 46017 Valencia, Spain. jlgorriz@senefro.org