Numerous epidemiological studies support the association of exposure to air pollution with adverse health effects leading to enhanced morbidity and mortality of considerable significance.1, 2 In fact, the World Health Organization ranks it as the 13th leading cause of worldwide mortality. Cumulative evidence over the last decade suggests that the largest portion of air pollution-related mortality is due to cardiovascular diseases,3 on the basis of which it has been proposed to be a “modifiable” novel cardiovascular risk factor of great importance. While air pollution is a complex mixture of compounds in the gaseous and particle phases, more evidence implicates the particulate matter (PM) components as responsible for a major portion of the cardiovascular effects.2, 4 The PM components are classified according to their aerodynamic diameter into size fractions such as PM10 (“thoracic” particles, < 10μm), PM2.5-10 (“coarse” particles, 2.5 to 10μm), PM2.5 (“fine” particles, < 2.5μm) and UFP (“ultrafine” particles, < 0.1μm).4 These PM of different sizes appear to carry different abilities to cause harmful effects and there is increasing debate about the notion that systemic cardiovascular effects could be favored by a smaller particle size.5
Thus, exposure to ambient PM leads to enhanced cardiovascular morbidity and mortality due to a myriad of acute and chronic effects. Acute exposure to PM has been associated with the triggering of acute myocardial infarctions,6 discharge of implanted automatic cardioverter defibrillators,7 hospitalizations for ischemic strokes, and decompensated congestive heart failure.8 Therefore, the article by Domínguez-Rodríguez et al.9 published in Revista Española de Cardiología is very important as it approached the question whether air pollution could preferentially associate with hospital admissions due to heart failure (HF) vs acute coronary syndromes (ACS), in a terciary university hospital in Tenerife, Spain. While exposure to ambient particulate could enhance the incidence of both HF and ACS, leading to increased hospital admissions due to both causes, the study design evaluated whether there were differences between the two types of admissions. There was a small variation in environmental exposure parameters which impeded the authors from conducting a time series analysis.
Air pollutants were estimated by the average concentrations of PM10, PM2.5, and gases (NO2, SO2, NO, O3 and CO) in μg/m3 from the previous day up to 7 days prior to the admission. UFP were estimated as the average particle numbers/μm3. Two main points were evident from their findings: a) subjects admitted for HF had been subjected to greater levels of ambient PM than subjects admitted for ACS, and b) the associations were only significant for the UFP fraction and NO2 but not for PM2.5 PM10 or other gases. Let's consider the first point first. This study suggests that ambient particulate could preferentially enhance admissions due to HF exacerbations over ACS admissions. On the other hand, ambient PM has mostly been associated with cardiovascular events of ischemic nature. For instance, data from the Cancer Prevention Study II (CPS-II) showed that while mortality risk was identified with all cardiovascular causes despite weak associations with respiratory diseases, the mortality due to ischemic heart disease increased by 18% per each increase of 10μg PM2.5/m3 vs a 13% increase in deaths due to dysrhythmias, HF, and cardiac arrest, all combined.3 Likewise, data from the Women's Health Initiative Study (WHIS)10 showed an even greater 121% increase in deaths due to definite cases of coronary artery disease (CAD) per each increase in 10μg PM2.5/m3. In addition, while the incidence of overall CAD events (myocardial infarction, revascularization, angina and CAD death) increased by 17% in the WHIS, HF events were not associated with PM2.5 exposure in that study.10 How do air pollutants cause systemic cardiovascular effects that can make a greater impact on mortality than do their local effects induced in the lungs? How could we explain the preferential effects on HF decompensation over ACS?
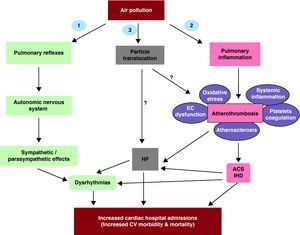
There are various mechanisms by which exposure to ambient PM could lead to cardiovascular systemic effects, including the involvement of 3 putative “general mediating” pathways (Figure 1): 1) Autonomic nervous system imbalance, 2) Induction of pulmonary and thereby systemic inflammation/oxidative stress via “spill-over” of mediators (eg, cytokines, activated white cells/platelets) into the systemic circulation, and 3) Access of particles or specific chemical constituents into the systemic circulation which thereby cause direct effects upon the heart and vasculature.11 All three pathways could potentially be involved in the induction of both acute and chronic effects with a degree of overlap that may be important to determine the specific effects, the timing of effects, and the dosing required to cause those effects. In addition, it is conceivable that all three pathways could participate and overlap in subjects admitted for both HF and ACS events. It is also possible that there could be a preponderance of one specific pathway depending on the type of effects. For instance, while cardiac dysrhythmias are likely to be predominantly mediated by pathway #1, HF decompensation and ACS events could be predominantly mediated by pathways #2 and #3 (Figure 1).
Figure 1. Potential mechanisms how exposure to particulate matter lead to increased cardiovascular diseases. Three main pathways could mediate particulate matter-related cardiovascular effects: 1) Induction of autonomic nervous system imbalance, 2) Development of pulmonary oxidative stress and inflammation with systemic “spill-over” of inflammatory mediators (eg, cytokines, activated cells), and 3) Translocation of particles and/or chemical constituents to the systemic circulation. ACS, acute coronary syndromes; CV, cardiovascular, EC, endothelial cells; HF, heart failure; IHD, ischemic heart disease.Modified from Araujo. 11
Since hospital admissions were categorized by the primary diagnosis on discharge, several admissions for one cause could have included the occurrence of the other type of event. For example, not only HF could develop in the setting of ischemic cardiomyopathy but decompensations leading to hospital admissions could be accompanied by acute ischemic events. Likewise, ACS events could lead to or be accompanied by HF decompensation. Given that secondary diagnoses were not taken into account, it is difficult to estimate the degree of crossover and until what extent there could have been a preferential underestimation of one type of diagnosis over another. In addition, this study was essentially comparative and aimed to determine preferential associations with one diagnosis over the other. The fact that PM10 or PM2.5 did not preferentially associate with either HF or ACS could have been due to similarly increased risk for both types of admissions. In support of this notion, a recent Italian study by Belleudi et al.12 showed that exposure to PM2.5 associated to a similar degree with hospital admissions due to both HF (2.4%) and ACS (2.3%) (Table 1). Another possibility is that the study may not have had enough power to detect preferential associations for PM10 or PM2.5 if the induced effects were too small, requiring larger studies for their detection. For instance, Wellenius et al.8 reported that PM10 exposure associated with a very small increase (0.72%) in hospital admissions for HF after studying 292 918 hospital admissions in 7 United States cities. Likewise, Dominici et al.15 demonstrated that admission rates for all types of cardiovascular events among 11.5 million U.S. Medicare enrollees aged > 65 years increased in association with a 10μg/m3 increase in PM2.5. Notably, the increase in risk was larger for HF (1.28%) than for ischemic heart disease (0.44%) or cerebrovascular disease (0.81%).15
Table 1. Studies Linking Exposure to Ultrafine Particles With Cardiac Hospital Admissions.
| Study | Number of cardiac hospital admissions | Environmental exposure parameters | Types of cardiac admissions | Mayor findings |
| Von Klot et al., 13 2005 | 6655 first hospital readmissions | PNCPM10Gases (CO, NO2, O3) | Acute MIAngina pectoris, HFDysrhythmia | Cardiac readmissions increased by 2.1% and 2.6% per each increase of 10μg/m3 of PM10 and 10 000 particles/cm3, respectively |
| Lanki et al., 14 2006 | 26 854 first MI admissions | PNCPM10Gases (CO, NO2, O3) | Acute MI | Hospitalizations for first MI increased by 0.5% per each increase of 10 000 particles/cm3 (lag 0). Associations were greater among fatal events and subjects < 75 years |
| Belleudi et al., 12 2010 | 90 056 cardiac hospital admissions | PNCPM10PM2.5 | HFACSOther cardiac causes | HF and ACS increased by 2.4% and 2.3% respectively per each increase of 10μg/m3 of PM2.5 (lag 0). HF increased by 1.7% per each increase of 9392 particles/cm3 (lag 0) |
| Domínguez-Rodríguez et al., 9 2011 | 3229 hospital admissions | PNCPM10PM2.5PM1Gases (CO, SO2, NO2, O3) | HFACS | UFP found to be a risk factor for HF admissions compared to admissions for ACS (odds ratio = 1.4) |
ACS, acute coronary syndromes; HF, heart failure; MI, myocardial infarction; PM, particulate matter; PNC, particle number concentration; UFP, ultrafine particles.
Recent studies have shown that PM associations with cardiovascular endpoints are stronger with PM2.5 than with PM10, in support of the notion that systemic cardiovascular effects are favored by the smaller particulate. From this perspective, UFP have been proposed to be the most active in inducing systemic effects. For instance, we and others have shown experimental and toxicological evidence that brings support to this notion5, 11; however, there is a paucity of epidemiological studies, partly due to the difficulties in capturing the true degree of exposure to UFP in population-based studies. Reliable measurement of UFP is difficult, partly because their concentrations are highly dependent on proximity to the source. In addition, routine air pollution monitoring does not include an assessment of UFP and there are no standards in place for their regulation. Several studies have used total particle number concentration (PNC) as a proxy metric, as did the authors in the current study, since the majority of particles fall within the nano-size range. It is interesting that despite the lack of preferential associations for both PM10 or PM2.5 and the imperfections of using total PNC to estimate UFP, exposure to UFP did associate with HF hospital admissions to a greater degree than with ACS hospitalizations. Furthermore, the authors also found a correlation with NO2 but not with any other gases. The NO2 and nano-size particle concentrations are very closely associated, likely because both are generated from combustion processes.16 These tight associations make it very difficult to differentiate between them in epidemiological studies and it is possible that the association of NO2 with hospital admissions for HF could have reflected the same association that was encountered with UFP.
Here again, the fact that UFP associated preferentially with HF admissions does not rule out the possibility that UFP could have also increased the risk for admissions due to ACS. Indeed, there are other reports about the role of UFP in increasing the risk for cardiac hospital admissions due to first acute myocardial infarctions14 and cardiac readmissions in survivors of myocardial infarction,13 listed in Table 1. However, the current results are consistent with the study by Belleudi et al.,12 as they found that total PNC showed an association with admissions for HF only but not for ACS (Table 1).
Indeed, the study from Domínguez-Rodríguez et al.9 suggests that PNC could be a more sensitive exposure parameter to detect differential effects between HF and ACS hospital admissions, which could be related to the potentially higher toxicity of UFP. How could UFP be more toxic than larger particles? First, UFP are much more numerous than bigger particles, accounting for more than 85% of the total PM2.5 particle number. Given their very small size, they account for a very small proportion of particle mass, which may explain why there could be differences related to UFP particle numbers but not to PM2.5 exposure mass.5 Second, UFP size has greater penetrability and diffusion into the lungs, leading to greater lung retention and possibly better cellular uptake and greater propensity to induce systemic effects.5 Third, UFP chemical composition is different from bigger particles. They may have a greater content of redox active compounds, such as prooxidative polycyclic aromatic hydrocarbons (PAHs) that could provide them with a greater prooxidative potential. In addition, their smaller size and greater surface-to-mass ratio may enable them to have greater bioavailability for the bioreactive chemicals (eg, PAHs, transition metals) on their large surface area, making them more accessible to the contact sites of cells.5 For all these reasons, it is not surprising that associations could have been detected with the number of particles and not with the exposure mass for PM2.5 or PM10. However, how the more active UFP could preferentially lead to HF over ACS hospital admissions may be related to how they differentially activate the various “general mediating” pathways mentioned above. Several questions of interest arise from the findings of the current study, such as: What is the precise mechanism(s) for UFP-induced HF decompensation? Would all patients with HF be equally susceptible to the effects of UFP? Is there a difference between ischemic vs non-ischemic cardiomyopathy? What are the UFP's active components responsible for their cardiovascular effects? Further studies are required to address these questions and many others.
In summary, the current study from Domínguez-Rodríguez et al.9 supports the association of exposure to UFP with hospital admissions for HF, adding new weight to its consideration as a cardiovascular risk factor. The study underscores the importance of the cardiovascular effects of air pollutants in a Spanish population. Further research is required to better understand the specific mechanisms by which PM of different sizes can lead to various cardiovascular effects. In addition, better parameters need to be developed to improve the assessment of UFP toxicity.
FUNDINGWriting of this editorial was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (RO1 Award ES016959 to Jesus A. Araujo).
CONFLICTS OF INTERESTNone declared.
.
Corresponding author: UCLA Division of Cardiology, 10833 Le Conte Avenue, CHS 43-264, Los Angeles, CA 90095, United States. JAraujo@mednet.ucla.edu