Atrial fibrillation is the most common sustained cardiac arrhythmia encountered in clinical practice, and since the prevalence is highly age-related, its public health impact in our aging societies is potentially enormous.1,2 Not only is our population aging, but the age-adjusted incidence and prevalence of atrial fibrillation is increasing, and these trends show no sign of abating.3,4,5,6 The prevalence of atrial fibrillation in the United States is approximately 2.3 million but this is likely a marked underestimation since many patients remain undiagnosed due to paroxysmal or asymptomatic disease or because of atypical symptoms. The underlying factors fueling this phenomenon are not well defined, but emerging trends in regard to increases in traditional and novel risk factors appear to play a prominent role, in particular, obesity and its associated comorbidities including type 2 diabetes, hypertension, and obstructive sleep apnea.
Stroke and systemic thromboembolism in patients with atrial fibrillationAtrial fibrillation is a major risk factor for stroke, increasing the risk of ischemic stroke approximately fivefold7,8,9,10 and accounting for approximately 45% of all embolic strokes in the United States (or approximately 100,000 strokes per year). Not only is age an independent and powerful predictor of stroke, but other risk factors for stroke in patients with atrial fibrillation also increase with age, including diabetes, congestive heart failure, hypertension, left ventricular dysfunction, and vascular disease.6 Of note, in patients less than 60years old without known risk factors the risk of stroke is extremely low, suggesting that it is not just the arrhythmia but the “company it keeps” that is responsible for systemic thromboembolism.11,12
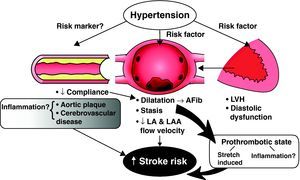
The pathophysiological mechanisms underlying stroke in patients with atrial fibrillation are multifactorial (Figure 1). It is well accepted that stasis in the left atrium and left atrial appendage leading to thrombus formation is likely a primary cause of systemic thromboembolism. Comorbidities in the elderly, including diastolic dysfunction, diabetes, hypertension, and aortic atherosclerosis, are associated with an increase in left atrial volume and perhaps an increased predisposition for left atrial stasis or thrombus formation.2,13,14,15,16 Left atrial volume overload and dilatation may in itself lead to a prothrombotic state via stretch-induced mechanisms and to endothelial dysfunction. In addition, the onset of atrial fibrillation may lead to activation of hemostatic factors.17 Several studies have suggested that atrial fibrillation induces a hypercoagulable state, and this has been noted in both paroxysmal and persistent atrial fibrillation17,18,19 (Figure 1). Moreover, increased plasma concentrations of markers of platelet activation (beta thromboglobulin, platelet factor IV, and soluble P-selection), increased plasma markers of thrombogenesis (thrombin–antithromben complexes), and evidence of endothelial dysfunction and damage (von Willebrand factor) have been demonstrated as independent correlates of thromboembolism.20,21
Figure 1. The role of hypertension and the risk of stroke in nonvalvular hypertension. Vascular disease and increased arterial stiffness may lead to left ventricular hypertrophy, diastolic dysfunction, and left atrial volume overload with stasis and thrombus formation. Other mechanisms may be the impact of atrial fibrillation and atrial stretch as contributors to a hypercoagulable state. Hypertension and valvular disease may also be a “risk marker” indicator for cerebrovascular, cerebral atherosclerosis, and an inflammatory “milieu”, but in themselves are not factors for stroke. AFib, atrial fibrillation; LA, left atrial; LAA, left atrial appendage; LVH, left ventricular hypertrophy.
In an unspecified proportion of patients, atrial fibrillation may be a surrogate or manifestation of increased aortic stiffness, diffuse atherosclerosis, and vascular disease. In these clinical subsets the putative cause of stroke and systemic embolism may be aortic atherosclerosis, coexisting cerebrovascular disease, and perhaps an inflammatory “milieu” (Figure 1). The demographic tide, resulting in aged societies in both the high and low-middle income countries, is inexorable and emphasizes the growing importance of stroke prevention in atrial fibrillation.
Risk stratification for thromboembolismA driving force underlying the recommendations for antiplatelet and anticoagulant therapy is the stratification of patients according to their risk of bleeding and the risk of stroke. This, in turn, has led to the publication of several different risk stratification schemes.22,23
In a large study of adults with atrial fibrillation, the predictive ability of 5 different risk scores were compared, including the widely used CHADS2 score.23 The different risk scores were comparable in their predictive ability, but the discriminative ability was uniformly poor with a C-statistic ranging from 0.56 to 0.62, and the proportion of patients judged to be at high, low, or intermediate risk varied widely across the schemes.
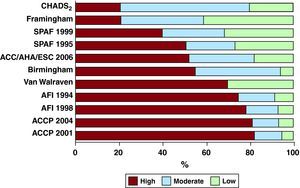
The Stroke Risk in Atrial Fibrillation Working Group analyzed 12 published schemes for stratifying risk in patients with nonvalvular atrial fibrillation.22 Five were based upon expert consensus; four schemes considered only clinical factors, whereas seven included echocardiographic variables. Moreover, the number of variables included varied from four to eight. Figure 2 illustrates the marked variability in the prediction of the category of risk. The conclusion from this working group was that there were substantial, clinically relevant differences amongst the published schemes, and all were far from optimal. Performance was particularly suboptimal in low-risk patients, and there is a lack of data on actual stroke rates in high-risk patients. There is also a lack of consensus on the role of congestive heart failure as a risk factor and the impact of the duration, severity, and treatment of key morbidities such as hypertension upon overall risk. The role of biomarkers reflecting the prothrombotic state as a contributing risk stratification factor has not been established.
Figure 2. Relative distribution of predicted stroke risk by applying 12 different risk stratification schemes to a representative cohort of atrial fibrillation patients. ACC, American College of Cardiology; ACCP, American College of Chest Physicians; AFI, Atrial Fibrillation Investigators; AHA, American Heart Association; ESC, European Society of Cardiology; SPAF, Stroke Prevention in Atrial Fibrillation. Reproduced from Stroke Risk in Atrial Fibrillation Working Group 22 with permission.
Nonetheless, and despite the known limitations of the CHADS2 risk score in predicting stroke risk and left atrial thrombus,24 it does appear that in patients on acetylsalicylic acid and cloplidogrel, the relationship between increasing scores and the risk of stroke is quite strong and clinically meaningful.25,26 In addition, it must be recognized that risk is a continuum and a CHADS2 score of 1 still implies an increased risk and possible benefit from oral anticoagulants. The recent European Society of Cardiology (ESC) guidelines for the management of atrial fibrillation incorporated the CHA2DS2-VASc risk score, which extends the CHADS2 schema by considering additional stroke risk.27 The score may be particularly helpful for patients with a CHADS2 score of 1, for whom the risk may be high enough to warrant oral anticoagulation (OAC) with 1 of the 3 additional “VASc” factors: age 65-74 (compared to <65), female sex, or vascular disease. The validation of this in other populations has been of interest and a recent large series suggested that among an elderly population the CHADS2 and CHA2DS2-VASc models had the best predictive value.28,29 Nonetheless, the clinical utility of the CHA2DS2-VASc risk score needs to be further validated.30 In conjunction with risk factors developed for the prediction of stroke, various bleeding risk scores have been validated for use in anticoagulated patients, again with considerable variability. A new simple bleeding risk score (HAS-BLED) based upon hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), age greater than 65, and concomitant drugs and alcohol use has been derived and, according to the ESC guidelines, a score of greater or equal to 3 indicates high risk for bleeding on anticoagulation therapy.31
Clearly, a priority for further research is the development of optimum and standardized schemes for primary prevention, perhaps utilizing biomarkers of thrombogenesis in addition to other parameters.
Evidence-based strategies for anti-platelet and antithrombotic therapy in atrial fibrillationThe indications for therapy to prevent stroke and non-central nervous system systemic thromboembolism in patients with atrial fibrillation are based upon an assessment of the absolute risk of such events and the potential for bleeding. These are addressed thoroughly by the current ESC and American College of Cardiology/American Heart Association (ACC/AHA) guidelines, but as is the case in many other clinical settings, therapy needs to be individualized and sound clinical judgment exercised.27,32 This requires a thorough appreciation of the “entire patient”, including comorbidities and compliance. With a CHADS2 score of 2 or higher, OAC is recommended; for a CHADS2 score of 1 either acetylsalicylic acid or OAC, and for a CHADS2 score of 0 either acetylsalicylic acid or nothing is reasonable. The CHA2DS2-VASc schema can be especially helpful for patients with CHADS2 score of 1, for whom an additional VASC factor may move them into a category warranting OAC.
Acetylsalicylic AcidA meta-analysis of 7 trials comparing acetylsalicylic acid with placebo demonstrated that acetylsalicylic acid was associated with a slightly reduced 19% (95% confidence interval [IC], -1% to -35%) incidence of stroke.33 The major effect appeared to be in reduction of nondisabling stroke. The most recent randomized trial of acetylsalicylic acid (150-200mg/dL) versus placebo was, however, completely neutral.34 The data in regard to dipyridamole are insufficient to justify a firm conclusion. In summary, it appears that there may be a benefit from acetylsalicylic acid, but the effect is small and the data are not conclusive.
Acetylsalicylic Acid Plus Clopidogrel Compared to Acetylsalicylic Acid AloneIn the large ACTIVE study, a randomized trial of 7554 patients with atrial fibrillation and 1 to 2 stroke risk factors, clopidogrel at 75mg per day and acetylsalicylic acid 75mg to 100mg per day was compared to acetylsalicylic acid alone (75mg to 100mg per day) in patients who were not candidates for warfarin therapy based upon physician and patient preference.35 After a period of 3.6years, clopidogrel plus acetylsalicylic acid significantly reduced the rate of the combined endpoint of first occurrence of stroke, non-central nervous system systemic embolism, myocardial infarction, and vascular death, with a relative risk of 0.89 (0.81 – 0.98), and this was primarily driven by a 28% relative risk reduction in stroke. This benefit came at a price of a 50% increase in major bleeding. This trial suggests nonetheless that dual antiplatelet therapy is an alternative in patients who cannot take warfarin, but the bleeding risks are similar to those of oral anticoagulants.
Adjusted Dose Warfarin Compared to Antiplatelet TherapyIn a meta-analysis of 29 randomized clinical trials including 28000 patients with non-valvular atrial fibrillation, warfarin therapy led to a 64% relative risk reduction in stroke (95% CI, 49% to 74%) compared with placebo or antiplatelet agents. Also importantly, warfarin was associated with a relative 26% reduction in all cause mortality (95% CI, 3% to 34%).33
There are a number of factors related to the randomized controlled trials that may affect generalization to the wider realm of clinical practice. The effects of warfarin may have been exaggerated by an “entry bias” limiting patients at higher risk of bleeding, the participation of expert centers, open label trials, and limited background therapy (thus inflating event rates). On the other hand, other factors may have diminished the warfarin effect in regard to clinical practice in that many patients were warfarin-naïve, some of the studies antedated the INR era and utilized variable prothrombin time ratios, and aggressive modification of risk factors could have reduced vascular events.
Adjusted Dose Warfarin Versus Acetylsalicylic Acid and ClopidogrelThe ACTIVE W trial included 6706 patients randomly assigned to a vitamin K antagonist (INR target, 2.0-3.0) or acetylsalicylic acid (75mg to 100mg per day), and clopidogrel 75mg daily.36 The trial was prematurely terminated because warfarin anticoagulation was clearly superior in terms of the primary endpoint, which was driven by stroke. Overall rates of bleeding with dual antiplatelet therapy were also higher. Of interest is a subsequent post hoc analysis from this trial which demonstrated that the benefit from oral anticoagulants was confined to those patients with a time in the therapeutic range (TTR) of 65% or greater, of whom the risk of primary event on acetylsalicylic acid and clopidogrel was more than doubled in comparison to warfarin.37
Low Dose Acetylsalicylic Acid and WarfarinThe “SPAF III” trial of 1044 patients unequivocally demonstrated that low dose warfarin (1.25mg per day or a target INR between 1.2 and 1.5) in combination with 300mg acetylsalicylic acid per day is clearly inferior to adjusted dose warfarin in terms of morbidity and mortality.38
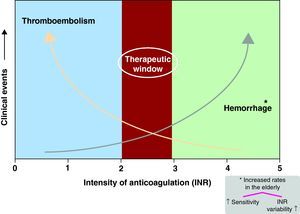
Limitations of vitamin K antagonistsThe limitations of vitamin K antagonists are well known, including a narrow therapeutic range, slow “onset and offset” of action (Figure 3), numerous food and drug interactions, the impact of concurrent illnesses in pharmacokinetics and pharmacodynamics, and the influence of genetic variability on clearance.39 The need for constant and frequent monitoring for anticoagulant effect and frequent dose adjustments introduces an element of inconvenience.
Figure 3. Illustrates the narrow therapeutic window of vitamin K antagonists with an increased risk of stroke when the INR falls below 2 and increased risk of bleeding with INR > 3-3.5. INR: international normalized ratio.
Of additional concern is the marked variability in the time that the INR is in the TTR and the impact of the quality of INR control in regard to the benefits of warfarin over antiplatelet therapy.37 In the SPORTIF II randomized trial of ximelagatran versus warfarin, at the end of 12weeks only 57% of patients were in the therapeutic range of an INR of 2-3.40 In a recent multinational randomized trial, distributions of the mean time in the therapeutic INR range varied from 44% to 77%. Home INR monitoring has had a positive but surprisingly small effect on the time in the TTR.41 Several recent reports have demonstrated a strong relationship between INR control and stroke in patients with atrial fibrillation.42,43 Despite these caveats, however, in a large integrated healthcare system in California, warfarin was shown to be very effective in preventing ischemic stroke in patients with atrial fibrillation, and the risk of intracranial hemorrhage was small.44 Nonetheless, despite the undeniable fact that warfarin is cheap and effective, it is not an easy drug to administer and seems to be widely disliked by physicians, patients, and the media.
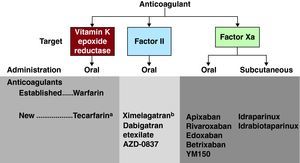
Alternatives to warfarin: a new ERA?The advent of alternatives to warfarin has engendered an enthusiastic response and in some quarters perhaps unreasonable expectations. Adopting new anticoagulant agents for clinical use is difficult, and there are substantial methodological challenges, but the last few years have witnessed considerable progress in the field.45,46 The investigative pathway followed by most new agents has generally begun with trials in patients with venous thromboembolism in which targets are the specific steps of coagulation, including initiation, propagation, and thrombin activity. The latter may promote thrombus-mediated activation of other coagulation factors. Subsequent and at times parallel trials have focused upon patients with atrial fibrillation and, in regard to some drugs, patients with acute coronary syndromes (Figure 4).
Figure 4. Established and new anticoagulants for stroke prevention in atrial fibrillation. aBypasses CYP450 pathway. bWithdrawn due to hepatotoxicity.
TecarfarinAs opposed to other agents that directly target the coagulation cascade, tecarfarin (ATI-5923) is a vitamin K epoxide reductase antagonist that has a mechanism of action identical to that of warfarin. The advantage is that it is metabolized by carboxylesterases and not the cytochrome P450 (CYP450) pathway. From a theoretical perspective this could reduce many of the food, drug, and genetic interactions that may underlie the variability in INRs, particularly during the first 3 months after the initiation of warfarin.
The first evaluation of this drug was recently performed in 66 patients in a 6-week to 12-week open-label study, and this suggested a modest (approximately 10%) improvement in the TTR in comparison to prior warfarin studies.47 A much larger study including a concurrent control warfarin arm will be required to assess efficacies, safety, and tolerability.39
Factor II (direct thrombin) inhibitorsThrombin plays a critical role in hemostasis (Figure 4), and this is mediated through its actions on several key pathways, including the conversion of fibrinogen to fibrin, amplification of the process of coagulation by activation of critical factors, direct activation of platelets, and clot stabilization by a number of independent mechanisms.48,49 Thrombin inhibition is understandably an attractive target, and the direct thrombin inhibitors are able to inactivate both soluble and clot-bound thrombin, a potentially important characteristic as release of the latter after lysis can trigger further thrombus growth.50
The first direct thrombin inhibitor to be approved in the clinical era was ximelagatran for patients with venous thromboembolism. Despite showing some promise in patients with atrial fibrillation, the drug was withdrawn from the market due to liver toxicity in February 2006.51
Dabigatran EtexilateDabigatran Etexilate (Pradaxa®) is an orally available small-molecule direct thrombin inhibitor which was approved in Europe in 2008 for venous thromboembolism prophylaxis after orthopedic surgery and in the United States and Canada in 2010 for stroke prophylaxis in patients with atrial fibrillation. It is a pro-drug that is converted into an active metabolite upon ingestion independent of CYP450; bioavailability is low, and the dose is twice daily. It is predominantly cleared by the kidneys (80%) by a rapid onset of action with an anticoagulant effect in about 1h but a half life of 12h to 17h. INR testing is not required. The pivotal open-label RE-LY trial in 18,113 patients with atrial fibrillation demonstrated that at a dose of 150mg twice per day. Dabigatran was associated with lower rates of stroke and systemic embolism, but similar rates of major bleeding in comparison to warfarin.52 At a dose of 110mg twice per day, rates of stroke and systemic embolism were similar to those associated with warfarin, but rates of major hemorrhage were lower. These positive findings were independent of the quality of INR control at participating sites.53 In patients with prior stroke or transient ischemic attacks, the effects of dabigatran on stroke with systemic embolism were similar to those associated with wafarin, but the bleeding rates were lower.54 For both doses of dabigatran, the relative risk of intracranial hemorrhage was reduced by 60% compared to warfarin.
In the United States, dabigatran 150mg twice per day has been approved, but in patients with poor renal function (creatinine clearance of 15-30ml/min) a lower dose of 75mg twice per day based upon pharmacodynamic modeling is recommended. Dabigatran is unquestionably an advance, but its approval has generated new questions:
1) The twice per day dosing may have disadvantages, including patient compliance.
2) The shorter duration of action may increase vulnerability to “rebound” if doses are missed.
3) Dyspepsia occurred in approximately 11% versus 5.8% on warfarin in the RE-LY trial.
4) The dosing in patients with renal dysfunction has not been substantiated by clinical trials.
5) There was an unexplained slightly higher rate of myocardial infarction with dabigatran than warfarin in the RE-LY trial, and it is uncertain as to whether this relates to the nonuse of acetylsalicylic acid in many patients, a direct effect of warfarin, and whether or not this increase in rates of myocardial infarction are clinically relevant.
6) Although bleeding rates have decreased, the lack of reversibility other than with hemodialysis could be a concern, particularly in patients undergoing surgery that entails a high risk of bleeding. In general, dabigatran should be withheld for 4 to 5 half-lives (60h-75h) prior to an invasive procedure. If patients experience bleeding, pressure support, blood transfusions, and in exceptional circumstances dialysis is indicated. The use of additional hemostatic agents including factor VIII inhibitor bypass activity (FEIBA) and activated factor VII have not been systematically studied in this setting, are underused, and could lead to rebound thrombosis.
7) Another unresolved issue is the cost of the drug, and this may differ according to patient versus societal costs, since in many countries the costs of INR testing are not born by the patient. Ideal candidates for dabigatran are patients with nonvalvular atrial fibrillation who are difficult to control with warfarin due to widely fluctuating INR levels, patients who live in remote areas, or when good anticoagulation control (e.g., home INR monitoring) is not possible.
Factor Xa is also an attractive target for new antithrombotic approaches, since factor Xa selectively acts upon the central protease common to both the intrinsic and extrinsic pathways. In this manner, the major and rate-limiting source of amplification of the coagulation cascade would be inhibited.48,49 Figure 4 demonstrates that several oral and subcutaneous antiXa agents are in development. In regard to atrial fibrillation the focus is upon the oral agents.
RivaroxabanRivaroxaban is a small-molecule factor Xa inhibitor that has been extensively evaluated. It is rapidly absorbed with high bioavailability, dosing is once daily, the half life is quite short (5h to 9h in healthy volunteers), but significantly longer in the elderly (9h to 13h), and clearance is one-third renal and two-thirds hepatic. It does not require INR monitoring.
The largest trial in atrial fibrillation (Randomized, Double-Blind Study Comparing Once Daily Oral Rivaroxaban With Adjusted-Dose Oral Warfarin for the Prevention of Stroke in Subjects With Non-Valvular Atrial Fibrillation [ROCKET-AF]) in 14 172 patients was presented at the 2010 AHA Scientific Sessions.55 Patients in this double-blind trial were at high risk, with 90% having a CHADS2 risk score of 3 or higher and about half with prior history of stroke or transient ischemic attack. Although the drug achieved noninferiority in comparison to warfarin, superiority was not achieved. Nonetheless, the results should be reviewed in a positive light in that these data do show that it is a safe and effective alternative to warfarin. Like Dabigatran, rivaroxaban resulted in a significantly lower risk of intracranial hemorrhage than warfarin.
Questions arising from this presentation are several: a) Are there differences in the effects of factor Xa inhibitors and direct thrombin inhibitors (dabigatran) and is a head-to-head trial feasible and/or likely?; b) Given the high-risk population, are the ROCKET-AF results more relevant for the high-risk population, and RE-LY for the lower risk population? Was the single daily dose optimal in ROCKET-AF, particularly in view of the drug's relatively short half life?; c) Are the results of ROCKET-AF more reliable due to the double-blind design of the trial?, and d) What is the impact of the lower rate of TTR (57.8%) in warfarin patients specifically in regard to the magnitude of the difference in outcomes between warfarin and rivaroxaban? Publications from this trial are awaited with interest.
ApixibanThis is a potent selective factor Xa inhibitor with a half life of 12h, bioavailability of 50%, predominantly non-renal clearance, and twice per day dosing. There are no known food-drug interactions and likely few drug-drug interactions. It has been evaluated extensively in trials of deep vein thrombosis and prophylaxis, with encouraging results. The Apixaban versus Acetylsalicylic Acid to Prevent Strokes (AVERROES) trial —presented at the ESC Annual Scientific Sessions in 2010— compared apixiban to acetylsalicylic acid in 5600 patients who were “unsuitable” for warfarin, for reasons that included either prior warfarin intolerance or physician or patient choice.54 The trial was terminated early because Apixiban significantly reduced the risk of stroke and systemic emboli with no incremental risk of bleeding.56
The largest apixiban trial in patients with atrial fibrillation is the Apixaban for Reduction In Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, which has completed enrollment of 18,206 patients. Results are expected in 2011 and will include outcomes in a substantial number of patients who are “warfarin naïve”.
Other oral factor Xa inhibitors include betrixaban, darexaban, and edoxaban, and these are currently the subject of ongoing phase II (betrixaban and darexaban) and phase III (edoxaban) trials. In regard to edoxaban, the Effective Anticoagulation With Factor Xa Generation in Atrial Fibrillation Trial (ENGAGE AF-TIMI 48 [NCT00781391]) is confined to patients with atrial fibrillation and a CHADS2 score of 2 or greater.47 Darexaban is currently the subject of another ongoing phase II study.49
In regard to the role of these agents in patients with acute coronary syndromes, this is outside the purview of this review. Nonetheless, caution is needed. We know that triple therapy with warfarin, acetylsalicylic acid, and Plavix increases bleeding risks, and why should this be different with other anticoagulants? In this respect, the APPRAISE II (Apixaban for Prevention of Acute Ischemic Safety Events II) trial with Apixiban in patients with acute coronary syndromes, most of whom were receiving dual antiplatelet therapy, was recently prematurely terminated due to an excess of bleeding.55
Nonpharmacologic approachesMechanical and in particular catheter-based approaches to occlusion of the left atrial appendage are an innovative and exciting avenue of stroke prevention. The success of these procedures is predicated upon the assumption that the overwhelming approach of embolic strokes in patients with atrial fibrillation emanates from thrombi in the left atrial appendage.57,58 A counter argument emphasizes the concept of atrial fibrillation as a vascular disease, particularly in the elderly.59 AF is associated with increased aortic stiffness, aortic and cerebrovascular atherosclerosis, and disturbances in platelet function and coagulation. In this setting there are multiple potential sources of embolic stroke, thus warranting systemic anticoagulation.1,59
In patients who cannot safely tolerate anticoagulants because of the risk of bleeding, left atrial appendage occlusion is an attractive option and a number of devices are under evaluation; the only randomized controlled trial (Randomized Prospective Trial of Percutaneous LAA Closure vs Warfarin for Stroke Prevention in Atrial Fibrillation [PROTECT-AF]) of the Watchman device is encouraging.60 In the near future we will likely witness new iterations of devices and increased safety as experience on the learning curve increases. The ultimate role of a device-based strategy will require a further trial, but for the present anticoagulation remains the standard of care.
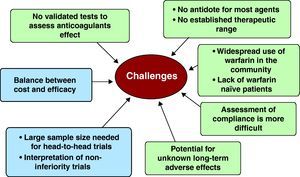
ConclusionsThe pathway to the development and approval of alternatives to warfarin is torturous, and there are many hurdles to be overcome (Figure 5).37,46 There remains considerable debate about the optimal clinical trial design, including issues such as open label versus double blind, the selection of appropriate endpoints, noninferiority trial design, considerations for the design of trials of superiority, and appropriate dosages. The development of utility measures to assess the impact of different endpoints upon the quality of life may be an important consideration in the patient's decision to start a new drug. Cost effectiveness at a societal and patient level is an entirely different but important issue.
Figure 5. Hurdles to be overcome and difficulties in the adoption of new anticoagulants.
The regulatory environment will play a major role in the design of clinical trials, and in regard to sample size these are likely to be large and costly given the relatively low event rates in warfarin-treated patients.46 Post-trial surveillance and registries are critical to a balanced long-term evaluation of a new drug in the wider world of clinical practice outside the more restricted milieu of the randomized controlled trial.
In the background but not to be ignored is the widespread underutilization of warfarin in eligible patients despite a wealth of performance measures and guidelines advocating it use in patients with moderate to severe risk of stroke.7,49,61 Moreover, even when it is used, its use is often suboptimal, with INRs outside of the target range.
In summary, the development and introduction into the clinical arena of new antithrombotic strategies is an important endeavor, and a worthy goal given the expanding public health problem posed by the combination of age and atrial fibrillation. Any therapy that removes the limitations of warfarin would be a welcome addition to the therapeutic armentarium. The approval of dabigatran in 2010, 56years after the Federal Drug Administration approved warfarin, marks the entering of a new era. In the near future, it is likely that we will have an array of new antithrombotic agents at our disposal in addition to established but inconvenient mainstay of oral anticoagulant therapy, namely vitamin K antagonists.
Conflicts of interestNone declared.
Corresponding author: Division of Cardiovascular Diseases, Department of Internal Medicine, Mayo Clinic, 200 First Street, SW, Gonda 5-368, Rochester, MN 55905-0001, United States. gersh.bernard@mayo.edu