Although of proven effectiveness, there are no data available on the patterns of aldosterone antagonists use in the setting of acute myocardial infarction.
MethodsThe REICIAM registry is a prospective study designed to provide data regarding the incidence and management of heart failure after acute myocardial infarction. The aim of the present analysis was to determine the patterns of aldosterone antagonists use in this situation.
ResultsFrom a total of 2703 patients with acute myocardial infarction, 416 (15.4%) were considered optimal candidates to receive aldosterone antagonists, but only 228 (54.8%) received the treatment. The independent factors associated with their administration were male sex (odds ratio=2.06; 95% confidence interval, 1.23-3.49; P=.006), absence of prior kidney failure (odds ratio=3.31; 95% confidence interval, 1.26-9.06; P=.02), presentation with ST elevation (odds ratio=2.01; 95% confidence interval, 1.21-3.35; P=.007) and the development of malignant arrhythmias (odds ratio=2.75; 95% confidence interval, 1.3-6.05; P=.009). The lower the ejection fraction, the higher the likelihood of receiving aldosterone antagonists. The major independent predictor for receiving aldosterone antagonists was the prescription of diuretics during hospitalization (odds ratio=7.11; 95%confidence interval, 3.72-14.23; P<.00001), but also treatment with clopidogrel, beta-blockers, and statins. Although patients treated with aldosterone antagonists had a higher risk profile, they had a better 30-day survival rate than untreated patients (88.3% and 77.7% respectively; P<.0001).
ConclusionsThe use of aldosterone antagonists in post-acute myocardial infarction is only 54.8% of the optimal candidates. Their use is associated with male sex, a higher risk profile, and the use of diuretics and other drugs of proven efficacy in secondary prevention.
Keywords
.
INTRODUCTIONSeveral clinical trials have demonstrated the effectiveness of therapies such as reperfusion, antiplatelet agents, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB-II), and statins in improving survival among patients who have had an acute myocardial infarction (AMI). The EPHESUS trial1 investigated the role of eplerenone, a selective aldosterone antagonist (AA), in patients with AMI complicated by left ventricular dysfunction and heart failure (HF). In this trial, the administration of eplerenone in addition to optimal medical therapy vs placebo reduced morbidity and mortality among the AMI patients with a left ventricle ejection fraction (LVEF) ≤40% and HF or diabetes mellitus. For this reason, the guidelines for the management of patients with AMI,2, 3 which were published after 2003, also include AAs as conventional therapy for patients who fulfill the selection criteria of the EPHESUS trial.
One of the greatest challenges in the treatment of AMI is transferring the information obtained from clinical trials into daily clinical practice. Several registries have analyzed the use of different therapies of proven efficacy in AMI during hospitalization and after discharge,4, 5 and many of them have shown that these therapies are underused.6, 7, 8, 9 However, none have investigated the use of AAs and the factors associated with their administration in the real-world context of AMI. The aim of this study was to determine the factors associated with AA use in AMI.
METHODSThe Spanish Registry of Heart Failure in Acute Myocardial Infarction (REICIAM) is designed to provide data on the incidence, management, and prognosis of patients with AMI complicated by HF in Spain. The registry is a prospective multicenter observational study of patients hospitalized for AMI between January 2006 and May 2008. The data were obtained at admission, during hospitalization, and at discharge. All public hospitals with cardiology departments were invited to participate. In total, there were 113 participating centers from 15 of the 17 autonomous communities of Spain. Of these, 83.7% were teaching centers accredited by the Ministry of Health, Social Policy, and Equality. The study included a participating hospital's first 10 consecutive patients in at least one month of the REICIAM study period who were at least 18 years of age, alive upon arrival at the hospital, and admitted for AMI, according to the current diagnostic criteria for recent or developing AMI of the European Society of Cardiology/American College of Cardiology published in 2000.10 These criteria are based on the typical rise and fall of cardiac troponin levels or the MB fraction of creatine kinase, in addition to at least one of the following: ischemic symptoms, development of Q waves on electrocardiogram, or electrocardiographic changes indicative of ischemia. When required, the researchers received approval to conduct this study from the regional ethics committees or from the hospital. Standard notebooks were used to record information on age, sex, characteristics of AMI, LVEF, comorbidities, inhospital management, and clinically significant complications. Postdischarge follow-up visits were scheduled. Assuming that the HF incidence of AMI patients is 20%,11 it was estimated that the sample size should be larger than 1388 patients, using a confidence level of 99%, precision of 3%, and an expected data loss of 15%.
From the population registry, we selected patients considered optimal candidates to receive AA (patients with documented LVEF ≤40%, HF symptoms at some point during initial hospitalization, or diabetes mellitus, and who had survived for more than 48h without contraindications). Serum creatinine levels >2.5mg/dL or potassium >5 mEq/L during hospitalization were considered contraindications for AA therapy.1 Patients considered eligible for AA therapy were classified into 2 groups: a) patients treated with AA, and b) patients not treated with eplerenone or spironolactone.
Data AnalysisThe main aim of the study was to analyze the factors associated with the use of AA in AMI patients and their indications. Of the optimal candidates to receive AA therapy, differences in demographic characteristics, medical history, clinical manifestations, and treatment were compared between the two study groups. Two-tailed Kruskal-Wallis test was used to compare continuous variables and Fisher χ2 test for discrete variables when required. The independent predictors of AA administration were determined by stepwise logistical regression. The variables included in the multivariate model were age, sex, history of kidney failure, type of AMI (with or without ST-segment elevation), HF diabetes mellitus, LVEF, serum potassium and creatinine concentrations, coronary angiography and type of hospital, as well as clinical variables; a P value ≤.1 was used as a cutoff for statistical significance in the univariate analysis. Other laboratory variables were excluded from the model. Goodness-of-fit of the final regression model was assessed using the Hosmer-Lemeshow test. The discriminative power of the model was assessed using the mean area under the receiver-operating characteristic curve (C statistic). The Kaplan-Meier method was used to estimate the 30-day survival rate. The log-rank test was used for between-group comparisons. Data analysis was performed using the SAS statistical software package (SAS Institute, Cary, North Carolina, United States).
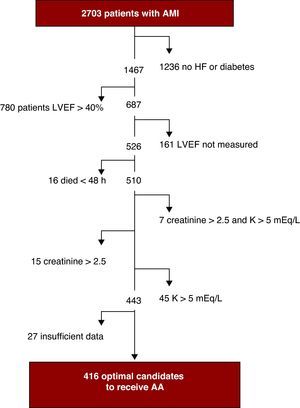
RESULTSTable 1 shows the baseline characteristics of the 2703 patients included in the registry and Table 2 shows their clinical presentation, course, and inhospital management. Mean follow-up time was 30±4 days. Figure 1 shows the process for selecting those considered suitable for treatment with AA. In total, there were 526 (19.5%) patients with documented LVEF ≤40% and HF or diabetes mellitus, 473 patients with LVEF ≤40% and HF (17%), and 416 patients (15.4%) considered optimal candidates for AA therapy. Of the optimal candidates, only 228 (54.8%) received treatment with eplerenone or spironolactone. Eplerenone was the drug of choice in 66% of the patients and spironolactone in 33%. The AA therapy began after a mean delay of 3 [interquartile range, 1-6] days from admission, with no difference in delay between eplerenone and spironolactone. In total, 231 (10.1%) of the 2287 patients classified as nonoptimal candidates received AA. Table 3 shows the differences between the optimal candidates who received AA and those who did not receive them.
Table 1. Baseline Characteristics of the Patients Included in the REICIAM Registry (N=2703).
| Age (years) | 67.4±13 |
| Women | 30.5 |
| Medical history | |
| Diabetes mellitus | 34.2 |
| Hypertension | 62.6 |
| Dyslipidemia | 51.2 |
| Current smoker | 30 |
| Former smoker | 28.6 |
| Previous ischemic heart disease | 29.7 |
| Previous myocardial infarction | 19.6 |
| Previous heart failure | 9.4 |
| Previous coronary revascularization | 12.9 |
| COPD | 16 |
| PVD | 11.8 |
| Previous TIA/Stroke | 10.1 |
| Previous CKF | 5.7 |
| Total cholesterol (mg/dL) | 181.8±45.8 |
| Plasma creatinine (mg/dL) | 1.1±0.53 |
| Serum potassium (mEq/L) | 4.28±0.56 |
CKF, chronic kidney failure; COPD, chronic obstructive pulmonary disease;; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Unless otherwise indicated, data are expressed as percentages or mean±standard deviation.
Table 2. Clinical Presentation, Course, and Inhospital Management of the Patients Included in the REICIAM Registry (N=2703).
| Clinical presentation | |
| SBP (mmHg) | 135.7±28.8 |
| DBP (mmHg) | 77.4±16.5 |
| Heart rate (bpm) | 81.2±20.9 |
| Time from onset of symptoms (h) | 4 [2-10] |
| NSTEMI | 51.2 |
| During hospitalization | |
| Appearance of Q wave | 51.2 |
| Heart failure | 36.5 |
| VT/VF | 7.7 |
| RV infarction | 4 |
| Markers of necrosis (peak enzyme levels) | |
| CK (U/L) | 604 [296-1299] |
| CK-MB (U/L) | 102 [49-233] |
| Troponin I (ng/mL) | 11.6 [3.2-32.8] |
| STEMI Procedures (n=1379) | |
| Coronary angiography | 73.8 |
| Percutaneous intervention | 64.4 |
| Primary angioplasty | 34.1 |
| Fibrinolysis | 36.9 |
| Without reperfusion | 28.7 |
| NSTEMI Procedures (n=1324) | |
| Coronary angiography | 64 |
| Percutaneous intervention | 45.3 |
| Pharmacological treatment | |
| Acetylsalicylic acid | 95.9 |
| Clopidogrel | 81.9 |
| Statins | 88.4 |
| Antiplatelet agents | 87.1 |
| Beta blockers | 79.1 |
| ACEI/ARB-II | 79.4 |
| Nitrates | 75.4 |
| Diuretics | 38 |
| Glycoprotein IIb/IIIa receptor antagonists | 20.3 |
| Calcium antagonists | 18 |
| Inotropic agents | 8.2 |
| Amiodarone | 8.1 |
| Digoxin | 6.1 |
ACEI, angiotensin-converting enzyme inhibitors; ARB-II, angiotensin II receptor blockers; CK, creatine kinase; CK-MB, MB fraction of creatine kinase; DBP, diastolic blood pressure; NSTEMI, non-ST-segment elevation acute myocardial infarction; SBP, systolic blood pressure; STEMI, ST-segment elevation acute myocardial infarction; VT/VF, ventricular tachycardia/ventricular fibrillation..
Unless otherwise indicated, data are expressed as percentages, mean±standard deviation or median [interquartile range].
Figure 1. Selection process of optimal candidates to receive aldosterone antagonists. AA, aldosterone antagonists; AMI, acute myocardial infarction; HF, heart failure; LVEF, left ventricular ejection fraction.
Table 3. Baseline Characteristics and Inhospital Management of Patients Treated or Not Treated With Aldosterone Antagonists Who Were Optimal Candidates to Receive Them.
| Patients treated (n=228) | Patients untreated (n=188) | P | |
| Age (years) | 71.5±10.6 | 72.2±11.2 | .52 |
| Women | 33.3 | 41.5 | .09 |
| Background | |||
| Diabetes mellitus | 54.4 | 54.3 | .93 |
| Hypertension | 30.4 | 30 | .93 |
| Dyslipidemia | 56.0 | 57.8 | .7 |
| Current smoker | 23.9 | 26.1 | .76 |
| Previous ischemic heart disease | 42.3 | 43.9 | .75 |
| Previous myocardial infarction | 33.2 | 33 | .96 |
| Previous TIA/stroke | 16.7 | 20.3 | .34 |
| Previous PVD | 19.8 | 16.8 | .42 |
| Serum creatinine (mg/dL) | 1.2±0.39 | 1.13±0.45 | .1 |
| Serum potassium (mg/dL) | 4.2±0.44 | 4.17±0.44 | .47 |
| Total cholesterol (mg/dL) | 187.9±49.6 | 175.1±50.5 | .02 |
| SBP (mmHg) | 129.3±27.3 | 129.1±28.2 | .95 |
| DBP (mmHg) | 76.6±17.1 | 73.1±15.2 | .04 |
| Heart rate (bpm) | 88.4±21.4 | 90.8±23.6 | .3 |
| ST-segment elevation | .02 | ||
| STEMI | 62.7 | 51.6 | |
| NSTEMI | 37.3 | 48.4 | |
| Heart failure during hospitalization | 93.9 | 82.5 | .0002 |
| Initial Killip class | .008 | ||
| I | 4.9 | 13.9 | |
| II | 83.5 | 78.9 | |
| III | 9.8 | 5.6 | |
| IV | 1.8 | 1.7 | |
| VT/VF | 21.5 | 11.2 | .005 |
| LVEF (%) | 31.9±6.6 | 33.1±6.6 | .05 |
| Maximum CK concentration (U/L) | 1396.3±1161.1 | 1138.9±1097.5 | .049 |
| Hospital management | |||
| Coronary angiography | 64.5 | 61.2 | .49 |
| PCI | 52.6 | 48.4 | .62 |
| CABG | 3.5 | 4.8 | .62 |
| Acetylsalicylic acid | 98.3 | 94.7 | .04 |
| Clopidogrel | 83.3 | 70.2 | .002 |
| Beta blockers | 86 | 69.2 | <.0001 |
| ACEI/ARB-II | 96.9 | 87.2 | .0002 |
| Diuretics | 91.2 | 64.4 | <.0001 |
| Statins | 89.9 | 78.7 | .002 |
| Nitrates | 93.9 | 87.9 | .04 |
| Antiplatelet agents | 78.1 | 77.7 | .92 |
| Inotropic agents | 18.9 | 11.7 | .04 |
| Digoxin | 13.4 | 15 | .52 |
| Amiodarone | 21.4 | 19.8 | .74 |
| Calcium antagonists | 11.8 | 13.3 | .68 |
| Type of hospital (accredited teaching center) | 83.3 | 87.8 | .2 |
ACEI, angiotensin-converting enzyme inhibitors; ARB-II, angiotensin II receptor blockers; CABG, coronary artery bypass graft; CK, creatine kinase; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation acute myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SBP, systolic blood pressure; STEMI, ST-segment elevation acute myocardial infarction; TIA, transient ischemic attack; VT/VF, ventricular tachycardia/ventricular fibrillation.
Data are expressed as percentages or mean±standard deviation.
Table 4 shows the odds ratio (OR) and 95% confidence intervals (95% CI) of all the variables included in the multivariate model. The independent predictors of candidate patients receiving AA were male sex (OR=2.06, 95% CI, 1.23-3.49, P=.006), absence of kidney failure (OR=3.31, 95% CI, 1.26-9.06, P=.02), presence of AMI with ST-segment elevation (OR=2.01, 95% CI, 1.21-3.35; P=.007), LVEF (per each 10% increase, OR=0.2, 95% CI, 0.06-0.62, P=.006), onset of ventricular fibrillation or ventricular tachycardia during hospitalization (OR=2.75, 95% CI, 1.3-6.05, P=.009) and concomitant treatment with diuretics (OR=7.11, 95% CI, 3.72-14.23, P<.00001), clopidogrel (OR=2.15, 95% CI, 1.14-4.13, P=.02), BB (OR=2.86, 95% CI, 1.57-5.29, P=.0007) and statins (OR=2.43, 95% CI, 1.21-5.17, P=.01) during hospitalization. The C statistic for this model was 0.81, indicating excellent discrimination.
Table 4. Odds Ratio for the Use of Aldosterone Antagonists in Patients With Indications for These Drugs, Adjusted for Potential Confounders.
| OR (95% CI) | P | |
| Age (each 10-year increase) | 2.59 (0.63-10.94) | .19 |
| Male sex | 2.06 (1.23-3.49) | .006 |
| Absence of previous kidney failure | 3.31 (1.26-9.06) | .02 |
| STEMI | 2.01 (1.21-3.35) | .007 |
| HF during hospitalization | 1.61 (0.7-3.79) | .26 |
| Diabetes mellitus | 1.35 (0.79-2.3) | .27 |
| LVEF (each 10% increase) | 0.2 (0.06-0.62) | .006 |
| Potassium (each 0.5 mEq/L increase) | 1.51 (0.53-4.34) | .44 |
| Creatinine (each 0.5 mg/dL increase) | 2.63 (0.75-9.51) | .13 |
| Coronary angiography | 0.78 (0.43-1.4) | .41 |
| VT/VF | 2.75 (1.3-6.05) | .009 |
| Treatment with diuretics | 7.11 (3.72-14.23) | <.00001 |
| Treatment with acetylsalicylic acid | 1.98 (0.5-8.69) | .34 |
| Treatment with clopidogrel | 2.15 (1.14-4.13) | .02 |
| Treatment with beta blockers | 2.86 (1.57-5.29) | .0007 |
| Treatment with ACEI/ARB-II | 2.43 (0.8-7.96) | .13 |
| Treatment with statins | 2.49 (1.21-5.17) | .01 |
| Treatment with nitrates | 1.84 (0.99-3.45) | .05 |
| Treatment with inotropic agents | 1.57 (0.77-3.27) | .22 |
| Type of hospital (accredited teaching center) | 0.56 (0.28-1.08) | .091 |
ACEI angiotensin-converting enzyme inhibitors; ARB-II, angiotensin II receptor blockers; 95% CI, 95% confidence interval; HF, heart failure; CI, confidence interval; LVEF, left ventricular ejection fraction; OR, odds ratio; STEMI, ST-segment elevation acute myocardial infarction; VT/VF, ventricular tachycardia/ventricular fibrillation.
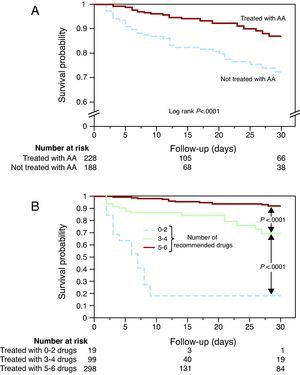
Candidates for receiving AA had a lower 30-day survival rate than those who were not eligible (84% vs 92%, respectively; P<.0001). Candidates for AA therapy who did not receive the medication had lower 30-day survival rates than those who received them (73% vs 87%, respectively, P<.0001) (Figure 2A) despite having a worse risk profile. The 30-day patient mortality was almost the same in teaching hospitals as in non-teaching hospitals (16.4% and 16.7%, respectively).
Figure 2. Kaplan-Meier survival curve of optimal candidates to receive treatment with aldosterone antagonists. A, comparison of survival between the patients who received aldosterone antagonists and those who did not receive them. B, probability of survival in relation to the number of recommended treatments used in patients with myocardial infarction, left ventricular dysfunction, and heart failure or diabetes (acetylsalicylic acid, clopidogrel, angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists, beta blockers, statins, or aldosterone antagonists). AA, aldosterone antagonist.
Since the administration of AA was associated with the administration of other therapies recommended for the secondary prevention of AMI, 30-day survival was analyzed according to the number of recommended therapies (acetylsalicylic acid, clopidogrel, ACEI/ARB-II, BB, statins, or AA) received. The fewer the number of recommended treatments in patients who were candidates to receive AA, the lower the 30-day survival rate (Figure 2B).
DISCUSSIONIt has been shown that AA therapy initiated early after AMI is superior to ACEI therapy alone to prevent post-infarction left ventricular remodeling12 and the development of HF.13 The EPHESUS trial1 demonstrated that eplerenone therapy in addition to standard therapy reduces mortality in a selected group of patients after AMI. In addition, the RALES trial14 demonstrated a reduction in mortality in patients with HF who received spironolactone in addition to conventional treatment. This finding led to a recommendation of AA use for a selected spectrum of patients in the clinical practice guidelines on the management of AMI2, 3 and HF.15, 16 Recently, the EMPHASIS-HF study demonstrated similar efficacy in a less symptomatic group of patients.17 Despite these findings, only one-third of patients admitted for HF who are eligible for AA therapy receive it.18 However, no information based on real-world experience is available on the use of AA in AMI. This is particularly important, given that in recent years some registries have demonstrated that many of the therapies with proven efficacy in AMI, such as antiplatelet agents, BBs, ACEIs/ARBs, or statins, are underused.6, 7, 8, 9, 19
Prescription Patterns of Aldosterone AntagonistsAccording to the EPHESUS trial,1 the ideal patients to receive AA include those with AMI associated with an LVEF ≤40% and clinical HF or diabetes mellitus, in the absence of contraindications. Of the 2703 patients included in the REICIAM registry, 416 were optimal to receive AA, and of these only 282 (54.8%) received eplerenone or spironolactone; this was the lowest rate of the recommended treatments. Acetylsalicylic acid, BB, and ACEI/ARB-II were used more frequently among those eligible for their use in this study and other series.20, 21, 22, 23 Furthermore, LVEF was not measured in 11% of patients with diabetes or HF (9.9% and 15.9% of patients in teaching and nonteaching hospitals, respectively; P=.03). It is possible that some of these patients also might have benefited from AA therapy.
When the REICIAM registry was designed, the hypothesis was that AAs would probably be used less than other therapies indicated in AMI complicated by HF and that their underuse could be due to concerns regarding their adverse effects (hyperkalemia, deterioration in kidney function, or antiandrogenic effects). This hypothesis is not fully supported by the results of the REICIAM registry. Although the underuse of AA was greater among patients with a prior history of kidney failure, creatinine and potassium concentrations during hospitalization were not independent predictors of AA use.
There was an increased tendency to use AA when the clinical presentation was more severe, as demonstrated by their increased use in patients with ST-segment elevation AMI and lower LVEF and in those who suffered inhospital ventricular fibrillation and/or ventricular tachycardias. This finding, although logical, is not scientifically justifiable; in the EPHESUS trial subgroup analysis, the only significant interaction between all-cause mortality and eplerenone therapy was its better effect in patients with higher blood pressure and serum creatinine <1.1mg/dL, previous hypertension, and the use of BB in combination with ACEIs/ARBs. However, no interactions were observed with LVEF or with diuretic therapy. The marked reduction in sudden death found in the EPHESUS trial1 could explain the higher rates of AA administration to patients with ventricular tachycardia and/or ventricular fibrillation during hospitalization. It is difficult to justify the significantly greater use of AA in men, but the fact that women receive inferior treatment with other recommended drugs has also been observed in many registries of AMI patients.6, 7, 8, 9, 19 The greatest predictor of AA use was diuretic therapy during hospitalization. This finding indicates that one of the aims of AA administration is to avoid hypokalemia. Compared to the patients who received AA he optimal candidates for AAs who did not receive them also received fewer pharmacological therapies of proven effectiveness in AMI, such as clopidogrel, BB, or statins. These results indicate that one of the reasons for their low use in some cases could be lack of knowledge regarding the clinical practice guidelines or skepticism regarding the results of the clinical trials. According to the current registry, the administration of AA to eligible patients was associated with greater survival despite their worse clinical profile. Nevertheless, given that the REICIAM registry is observational and thus open to several biases, we cannot conclude that the improved survival rates are due to the administration of this therapy. It was also confirmed that the lower use of recommended secondary prevention therapies had an impact on prognosis. Until now, most training programs developed by the Spanish Society of Cardiology for the management of AMI have been aimed at improving antithrombotic therapy and increasing the number of coronary angiographies and the use of BB, statins, and ACEI. These programs appear to have improved the administration of these drugs in just 3 years, if the results of the MASCARA (Manejo del Síndrome Coronario Agudo Registro Actualizado) registry11 are compared to the REICIAM registry. These training programs should probably include the aim of improving the use of AA and increasing the number of echocardiographic studies in the attempt to identify potential candidates.
The main limitation of the REICIAM registry is that it was not population based and that the participating hospitals were included on a voluntary basis. Furthermore, no quality controls were conducted to analyze the risk of possible selection biases. However, the incidence of HF or LVEF <40% among the patients with AMI in the REICIAM registry does not markedly differ from that of the population-based MASCARA study.11 The possibility remains that the prescription of AA is also associated with a variable not included in the analysis, such as comorbidities or factors related to the group of specialists who attended the patient during the hospitalization.
CONCLUSIONSThe results of this observational study demonstrate that AAs are used in half of the patients admitted for AMI who are considered optimal candidates for their administration. Their underuse could be due to their being perceived as less useful in candidates at lower risk and to a lack of knowledge about the indications for these agents and other recommended drugs.
FUNDINGAn unrestricted grant from Pfizer, Madrid, Spain.
CONFLICTS OF INTERESTDr. Esteban López-de-Sá and Dr. Manuel Anguita declare having received research grants and consulting and teaching fees from Pfizer.
Acknowledgements
We would like to thank all those who collaborated in the REICIAM registry, particularly those in charge of data collection.
Appendix A. REICIAM research groupCoordinators: Dr. E. López-de-Sá and Dr. A. Martinez.
Researchers: T. Abbas Abbas; M. Ali Al Salem Ahmed; P. Álvarez García; C. Amador Gil; P. Ancillo García; M. Anguita; F.J. Andrade de la Cal; M.A. Aparici Feal; J.C. Arias Castaño; A. Ariza Sole; J. Balaguer Recena; R. Bangueses Quintana; J.A. Barrabes Riu; V. Barriales Álvarez; J. Belchi Navarro; A. Benedicto Buendía; D. Bierge Valero; M.T. Blasco Peiro; A. Castilla Núñez; C. Cerdán Sánchez; C. Cerdeyra Lombardini; L.M. Ceresuela Eito; J.L. Colomer Marti; J. Comin Colet; J. Cortina Nicolás; J. Cosin Sales; J.M. Cucullo López; A. Curcio Ruigómez; A. Charles Chevannes; C. Daniel Riesco; L. de la Fuente Galan; F. de la Guía Galipienso; J. del Cazo Cativiela; L.V. Díaz Carretero; D. Dobarro; L.J. Domenech Delgado; L. Facila Rubio; D. Fernández Berges; J.M. Fernández Rodríguez; R. Freixa Pamias; J. Furundarena Zubiria; E. García; J.M. García Boldu; M. García García; M. García Martinez; H. García Delgado; I.P. Garrido Bravo; H. Gervas Pabon; A. Gimenez Agüera; J.A. González Brito; M.R. González Fernández; J.J. González Ferrer; M. González Ortega; A.E. Gordillo Higuero; J. Guardiola Tey; R. Guma González; F. Gutiérrez Marcos; P. Herrejon Silvestre; M.M. Izquierdo Gómez; R. Izquierdo González; J.D. Jiménez Delgado; M. Latorre López; J.A. Lomban Villanueva; L. López Barreiro; J.M. López de la Iglesia; D. López Gómez; J. López Martínez; T. Lozano Palencia; M.F. Llano Cardenal; D. Macia Pajares; F. Martín Herrero; J. Martín Pastor; L.V. Martínez Dolz; C. Martínez Useros; A. Mauri Plana; J. Mayordomo López; V. Montagud Balaguer; M.J. Montero Plaza; F.J. Monzón Lomas; M. Morillas Bueno; J.J. Muñoz Gil; P. Pabon Osuna; E. Paredes Galán; D.A. Pascual Figal; G. Pastor Baez; M.A. Paz Bermejo; M. Peraire Navarro; H. Pérez Hernandez; F. Planas Ayma; J.B. Polanco García; Y. Porras Ramos; J.J. Poveda Sierra; I. Ramio Viñets; J.R. Rey Blas; P. Rigueiro Veloso; A. Rius Davi; F. Rodon Lluis; M. Rodríguez González; F. Roncales; A. Rosello Serralta; F. Ruiz Martínez Corbalan; F. Ruiz Rejon; A. Saez González; A. Saez Jiménez; A. Salcedo Arruti; D. San Miguel; M.A. Sánchez Corral; A. Sánchez Grande; J.L. Santos Iglesias; J.J. Sanz Hernanz; M. Sanz Julve; L. Silva Melchor; C. Soriano Navarro; S. Temprano Vázquez; F. Teruel Carrillo; T. Torres Ramos; I. Ureña Montilla; A. Urrutia de Diego; G. Vázquez Oliva; B. Vega Hernández; P. Vigil Escalera; C. Villar Mariscal; J.C. Yañez Wonenburger; J. Zumalde Otegui.
Received 18 December 2010
Accepted 9 June 2011
Corresponding author: Unidad de Cuidados Cardiacos Agudos, Servicio de Cardiología, Hospital Universitario La Paz, P.o de la Castellana 261, 28043 Madrid, Spain. e.lopezdesa@terra.es